Biology:Dopamine transporter
 Generic protein structure example |
The dopamine transporter (DAT) also (sodium-dependent dopamine transporter) is a membrane-spanning protein coded for in the human by the SLC6A3 gene, (also known as DAT1), that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopamine into vesicles for storage and later release. Dopamine reuptake via DAT provides the primary mechanism through which dopamine is cleared from synapses, although there may be an exception in the prefrontal cortex, where evidence points to a possibly larger role of the norepinephrine transporter.[1]
DAT is implicated in a number of dopamine-related disorders, including attention deficit hyperactivity disorder, bipolar disorder, clinical depression, eating disorders, and substance use disorders. The gene that encodes the DAT protein is located on chromosome 5, consists of 15 coding exons, and is roughly 64 kbp long. Evidence for the associations between DAT and dopamine related disorders has come from a type of genetic polymorphism, known as a variable number tandem repeat, in the SLC6A3 gene, which influences the amount of protein expressed.[2]
Function
DAT is an integral membrane protein that removes dopamine from the synaptic cleft and deposits it into surrounding cells, thus terminating the signal of the neurotransmitter. Dopamine underlies several aspects of cognition, including reward, and DAT facilitates regulation of that signal.[3]
Mechanism
DAT is a symporter that moves dopamine across the cell membrane by coupling the movement to the energetically-favorable movement of sodium ions moving from high to low concentration into the cell. DAT function requires the sequential binding and co-transport of two Na+ ions and one Cl− ion with the dopamine substrate. The driving force for DAT-mediated dopamine reuptake is the ion concentration gradient generated by the plasma membrane Na+/K+ ATPase.[4]
In the most widely accepted model for monoamine transporter function, sodium ions must bind to the extracellular domain of the transporter before dopamine can bind. Once dopamine binds, the protein undergoes a conformational change, which allows both sodium and dopamine to unbind on the intracellular side of the membrane.[5]
Studies using electrophysiology and radioactive-labeled dopamine have confirmed that the dopamine transporter is similar to other monoamine transporters in that one molecule of neurotransmitter can be transported across the membrane with one or two sodium ions. Chloride ions are also needed to prevent a buildup of positive charge. These studies have also shown that transport rate and direction is totally dependent on the sodium gradient.[6]
Because of the tight coupling of the membrane potential and the sodium gradient, activity-induced changes in membrane polarity can dramatically influence transport rates. In addition, the transporter may contribute to dopamine release when the neuron depolarizes.[6]
DAT–Cav coupling
Preliminary evidence suggests that the dopamine transporter couples to L-type voltage-gated calcium channels (particularly Cav1.2 and Cav1.3), which are expressed in virtually all dopamine neurons.[7] As a result of DAT–Cav coupling, DAT substrates that produce depolarizing currents through the transporter are able to open calcium channels that are coupled to the transporter, resulting in a calcium influx in dopamine neurons.[7] This calcium influx is believed to induce CAMKII-mediated phosphorylation of the dopamine transporter as a downstream effect;[7] since DAT phosphorylation by CAMKII results in dopamine efflux in vivo, activation of transporter-coupled calcium channels is a potential mechanism by which certain drugs (e.g., amphetamine) trigger neurotransmitter release.[7]
Protein structure
The initial determination of the membrane topology of DAT was based upon hydrophobic sequence analysis and sequence similarities with the GABA transporter. These methods predicted twelve transmembrane domains (TMD) with a large extracellular loop between the third and fourth TMDs.[8] Further characterization of this protein used proteases, which digest proteins into smaller fragments, and glycosylation, which occurs only on extracellular loops, and largely verified the initial predictions of membrane topology.[9] The exact structure of the Drosophila melanogaster dopamine transporter (dDAT) was elucidated in 2013 by X-ray crystallography.[10]
Location and distribution
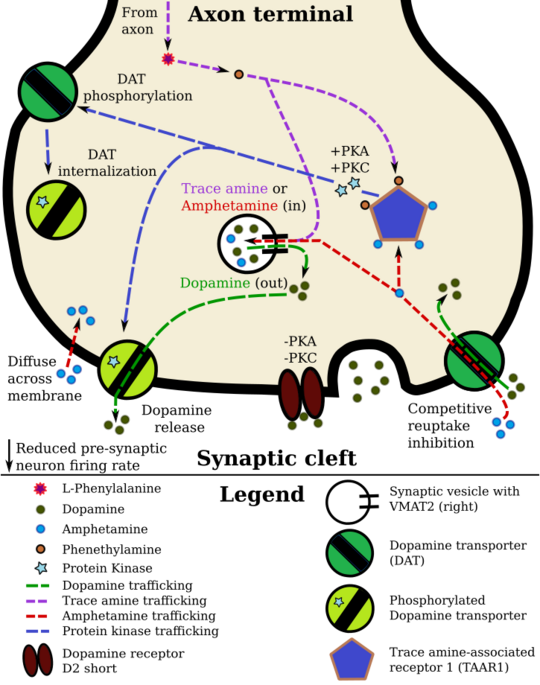
Pharmacodynamics of amphetamine in a dopamine neuron
|
Regional distribution of DAT has been found in areas of the brain with established dopaminergic circuitry, including the nigrostriatal, mesolimbic, and mesocortical pathways.[18] The nuclei that make up these pathways have distinct patterns of expression. Gene expression patterns in the adult mouse show high expression in the substantia nigra pars compacta.[19]
DAT in the mesocortical pathway, labeled with radioactive antibodies, was found to be enriched in dendrites and cell bodies of neurons in the substantia nigra pars compacta and ventral tegmental area. This pattern makes sense for a protein that regulates dopamine levels in the synapse.
Staining in the striatum and nucleus accumbens of the mesolimbic pathway was dense and heterogeneous. In the striatum, DAT is localized in the plasma membrane of axon terminals. Double immunocytochemistry demonstrated DAT colocalization with two other markers of nigrostriatal terminals, tyrosine hydroxylase and D2 dopamine receptors. The latter was thus demonstrated to be an autoreceptor on cells that release dopamine. TAAR1 is a presynaptic intracellular receptor that is also colocalized with DAT and which has the opposite effect of the D2 autoreceptor when activated;[11][20] i.e., it internalizes dopamine transporters and induces efflux through reversed transporter function via PKA and PKC signaling.
Surprisingly, DAT was not identified within any synaptic active zones. These results suggest that striatal dopamine reuptake may occur outside of synaptic specializations once dopamine diffuses from the synaptic cleft.
In the substantia nigra, DAT is localized to axonal and dendritic (i.e., pre- and post-synaptic) plasma membranes.[21]
Within the perikarya of pars compacta neurons, DAT was localized primarily to rough and smooth endoplasmic reticulum, Golgi complex, and multivesicular bodies, identifying probable sites of synthesis, modification, transport, and degradation.[22]
Genetics and regulation
The gene for DAT, known as DAT1, is located on chromosome 5p15.[2] The protein encoding region of the gene is over 64 kb long and comprises 15 coding segments or exons.[23] This gene has a variable number tandem repeat (VNTR) at the 3’ end (rs28363170) and another in the intron 8 region.[24] Differences in the VNTR have been shown to affect the basal level of expression of the transporter; consequently, researchers have looked for associations with dopamine-related disorders.[25]
Nurr1, a nuclear receptor that regulates many dopamine-related genes, can bind the promoter region of this gene and induce expression.[26] This promoter may also be the target of the transcription factor Sp-1.
While transcription factors control which cells express DAT, functional regulation of this protein is largely accomplished by kinases. MAPK,[27] CAMKII,[16][17] PKA,[11] and PKC[17][28] can modulate the rate at which the transporter moves dopamine or cause the internalization of DAT. Co-localized TAAR1 is an important regulator of the dopamine transporter that, when activated, phosphorylates DAT through protein kinase A (PKA) and protein kinase C (PKC) signaling.[11][29] Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces reverse transporter function (dopamine efflux).[11][30] Dopamine autoreceptors also regulate DAT by directly opposing the effect of TAAR1 activation.[11]
The human dopamine transporter (hDAT) contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[31][32][33] In contrast, the human serotonin transporter (hSERT) and human norepinephrine transporter (hNET) do not contain zinc binding sites.[33] Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of attention deficit hyperactivity disorder.[34]
Biological role and disorders
The rate at which DAT removes dopamine from the synapse can have a profound effect on the amount of dopamine in the cell. This is best evidenced by the severe cognitive deficits, motor abnormalities, and hyperactivity of mice with no dopamine transporters.[35] These characteristics have striking similarities to the symptoms of ADHD.
Differences in the functional VNTR have been identified as risk factors for bipolar disorder[36] and ADHD.[37][38] Data has emerged that suggests there is also an association with stronger withdrawal symptoms from alcoholism, although this is a point of controversy.[39][40] An allele of the DAT gene with normal protein levels is associated with non-smoking behavior and ease of quitting.[41] Additionally, male adolescents particularly those in high-risk families (ones marked by a disengaged mother and absence of maternal affection) who carry the 10-allele VNTR repeat show a statistically significant affinity for antisocial peers.[42][43]
Increased activity of DAT is associated with several different disorders, including clinical depression.[44]
Mutations in DAT have been shown to cause dopamine transporter deficiency syndrome, an autosomal recessive movement disorder characterized by progressively worsening dystonia and parkinsonism.[45]
Pharmacology
The dopamine transporter is the target of substrates, dopamine releasers, transport inhibitors and allosteric modulators.[46][47]
Cocaine blocks DAT by binding directly to the transporter and reducing the rate of transport.[8] In contrast, amphetamine enters the presynaptic neuron directly through the neuronal membrane or through DAT, competing for reuptake with dopamine. Once inside, it binds to TAAR1 or enters synaptic vesicles through VMAT2. When amphetamine binds to TAAR1, it reduces the firing rate of the postsynaptic neuron and triggers protein kinase A and protein kinase C signaling, resulting in DAT phosphorylation. Phosphorylated DAT then either operates in reverse or withdraws into the presynaptic neuron and ceases transport. When amphetamine enters the synaptic vesicles through VMAT2, dopamine is released into the cytosol.[11][12] Amphetamine also produces dopamine efflux through a second TAAR1-independent mechanism involving CAMKIIα-mediated phosphorylation of the transporter, which putatively arises from the activation of DAT-coupled L-type calcium channels by amphetamine.[7]
The dopaminergic mechanisms of each drug are believed to underlie the pleasurable feelings elicited by these substances.[3]
Interactions
Dopamine transporter has been shown to interact with:
Apart from these innate protein-protein interactions, recent studies demonstrated that viral proteins such as HIV-1 Tat protein interacts with the DAT[52][53] and this binding may alter the dopamine homeostasis in HIV positive individuals which is a contributing factor for the HIV-associated neurocognitive disorders.[54]
See also
References
- ↑ "Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals". Journal of Neurochemistry 55 (3): 1067–70. September 1990. doi:10.1111/j.1471-4159.1990.tb04599.x. PMID 2117046.
- ↑ 2.0 2.1 "Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR". Genomics 14 (4): 1104–6. December 1992. doi:10.1016/S0888-7543(05)80138-7. PMID 1478653.
- ↑ 3.0 3.1 "Predictive reward signal of dopamine neurons". Journal of Neurophysiology 80 (1): 1–27. July 1998. doi:10.1152/jn.1998.80.1.1. PMID 9658025.
- ↑ "Plasma membrane monoamine transporters: structure, regulation and function". Nature Reviews. Neuroscience 4 (1): 13–25. January 2003. doi:10.1038/nrn1008. PMID 12511858.
- ↑ "Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants". The Journal of Neuroscience 17 (3): 960–74. February 1997. doi:10.1523/JNEUROSCI.17-03-00960.1997. PMID 8994051.
- ↑ 6.0 6.1 "A model of the sodium dependence of dopamine uptake in rat striatal synaptosomes". Neurochemical Research 18 (8): 927–36. August 1993. doi:10.1007/BF00998279. PMID 8371835.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Amphetamine activates calcium channels through dopamine transporter-mediated depolarization". Cell Calcium 58 (5): 457–66. November 2015. doi:10.1016/j.ceca.2015.06.013. PMID 26162812. "One example of interest is CaMKII, which has been well characterized as an effector of Ca2+ currents downstream of L-type Ca2+ channels [21,22]. Interestingly, DAT is a CaMKII substrate and phosphorylated DAT favors the reverse transport of dopamine [48,49], constituting a possible mechanism by which electrical activity and L-type Ca2+ channels may modulate DAT states and dopamine release. ... In summary, our results suggest that pharmacologically, S(+)AMPH is more potent than DA at activating hDAT-mediated depolarizing currents, leading to L-type Ca2+ channel activation, and the S(+)AMPH-induced current is more tightly coupled than DA to open L-type Ca2+ channels.".
- ↑ 8.0 8.1 "Cloning and expression of a cocaine-sensitive rat dopamine transporter". Science 254 (5031): 578–9. October 1991. doi:10.1126/science.1948035. PMID 1948035. Bibcode: 1991Sci...254..578K.
- ↑ "Dopamine transporter ligand binding domains. Structural and functional properties revealed by limited proteolysis". The Journal of Biological Chemistry 271 (35): 21672–80. August 1996. doi:10.1074/jbc.271.35.21672. PMID 8702957.
- ↑ "X-ray structure of dopamine transporter elucidates antidepressant mechanism". Nature 503 (7474): 85–90. November 2013. doi:10.1038/nature12533. PMID 24037379. Bibcode: 2013Natur.503...85P.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry 116 (2): 164–76. January 2011. doi:10.1111/j.1471-4159.2010.07109.x. PMID 21073468.
- ↑ 12.0 12.1 12.2 "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Annals of the New York Academy of Sciences 1216 (1): 86–98. January 2011. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013. Bibcode: 2011NYASA1216...86E.
- ↑ "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia 6 (3): 123–148. August 2016. doi:10.1016/j.baga.2016.02.001. PMID 27141430. "Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.".
- ↑ "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. July 2011. doi:10.3389/fnsys.2011.00056. PMID 21772817. "Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.".
- ↑ "TAAR1". GenAtlas. University of Paris. 28 January 2012. http://genatlas.medecine.univ-paris5.fr/fiche.php?symbol=TAAR1. Retrieved 29 May 2014. " • tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)"
- ↑ 16.0 16.1 "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron 83 (2): 404–416. July 2014. doi:10.1016/j.neuron.2014.05.043. PMID 25033183. "AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012).".
- ↑ 17.0 17.1 17.2 "Mechanisms of dopamine transporter regulation in normal and disease states". Trends in Pharmacological Sciences 34 (9): 489–96. September 2013. doi:10.1016/j.tips.2013.07.005. PMID 23968642. "AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].".
- ↑ "Immunocytochemical localization of the dopamine transporter in human brain". The Journal of Comparative Neurology 409 (1): 38–56. June 1999. doi:10.1002/(SICI)1096-9861(19990621)409:1<38::AID-CNE4>3.0.CO;2-1. PMID 10363710.
- ↑ "Study of gene function based on spatial co-expression in a high-resolution mouse brain atlas". BMC Systems Biology 1: 19. April 2007. doi:10.1186/1752-0509-1-19. PMID 17437647.
- ↑ "Trace amine receptor: TA1 receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. 19 July 2016. http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=364.
- ↑ "The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons". The Journal of Neuroscience 16 (2): 436–47. January 1996. doi:10.1523/JNEUROSCI.16-02-00436.1996. PMID 8551328.
- ↑ "Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra". The Journal of Comparative Neurology 388 (2): 211–27. November 1997. doi:10.1002/(SICI)1096-9861(19971117)388:2<211::AID-CNE3>3.0.CO;2-4. PMID 9368838.
- ↑ "Structure and organization of the gene encoding human dopamine transporter". Gene 195 (1): 11–8. August 1997. doi:10.1016/S0378-1119(97)00131-5. PMID 9300814.
- ↑ "A 40-nucleotide repeat polymorphism in the human dopamine transporter gene". Human Genetics 91 (4): 405–6. May 1993. doi:10.1007/BF00217369. PMID 8500798.
- ↑ "Polymorphisms in the 3'-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression". Molecular Psychiatry 7 (1): 44–55. 2002. doi:10.1038/sj/mp/4000921. PMID 11803445.
- ↑ "Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism". Journal of Neurochemistry 76 (5): 1565–72. March 2001. doi:10.1046/j.1471-4159.2001.00181.x. PMID 11238740.
- ↑ "Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity". The Journal of Neuroscience 23 (24): 8480–8. September 2003. doi:10.1523/JNEUROSCI.23-24-08480.2003. PMID 13679416.
- ↑ "Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter". Synapse 30 (1): 79–87. September 1998. doi:10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. PMID 9704884.
- ↑ "Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors". Genomics 85 (3): 372–85. March 2005. doi:10.1016/j.ygeno.2004.11.010. PMID 15718104.
- ↑ "International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature". Pharmacological Reviews 61 (1): 1–8. March 2009. doi:10.1124/pr.109.001107. PMID 19325074.
- ↑ "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Review of Neurotherapeutics 8 (4): 611–25. April 2008. doi:10.1586/14737175.8.4.611. PMID 18416663. "Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.".
- ↑ "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron 69 (4): 628–49. February 2011. doi:10.1016/j.neuron.2011.02.010. PMID 21338876. "They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).".
- ↑ 33.0 33.1 "The role of zinc ions in reverse transport mediated by monoamine transporters". The Journal of Biological Chemistry 277 (24): 21505–13. June 2002. doi:10.1074/jbc.M112265200. PMID 11940571. "The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET).".
- ↑ "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". Journal of the American Academy of Child and Adolescent Psychiatry 51 (10): 1003–1019.e20. October 2012. doi:10.1016/j.jaac.2012.08.015. PMID 23021477. "With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110".
- ↑ "Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity". Science 283 (5400): 397–401. January 1999. doi:10.1126/science.283.5400.397. PMID 9888856. Bibcode: 1999Sci...283..397G.
- ↑ "Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder". American Journal of Medical Genetics 105 (2): 145–51. March 2001. doi:10.1002/1096-8628(2001)9999:9999<::AID-AJMG1161>3.0.CO;2-8. PMID 11304827.
- ↑ "A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3'-UTR of dopamine transporter gene and attention deficit hyperactivity disorder". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 144B (4): 541–50. June 2007. doi:10.1002/ajmg.b.30453. PMID 17440978.
- ↑ Seymari, Abbas; Naseh, Ashkan; Rezaei, Sajjad; Salehi, Zivar; Kousha, Maryam (8 December 2023). "The Relationship between Gene SLC6A3 Variable Number of Tandem Repeat (VNTR) and Attention-Deficit / Hyperactivity Disorder". Iranian Journal of Psychiatry 4 (1): 99–106. doi:10.18502/ijps.v19i1.14345. https://ijps.tums.ac.ir/index.php/ijps/article/view/3489.
- ↑ "Allelic association of a dopamine transporter gene polymorphism in alcohol dependence with withdrawal seizures or delirium". Biological Psychiatry 41 (3): 299–304. February 1997. doi:10.1016/S0006-3223(96)00044-3. PMID 9024952.
- ↑ "Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism". Molecular Psychiatry 4 (6): 552–7. November 1999. doi:10.1038/sj.mp.4000562. PMID 10578237.
- ↑ "Genetic polymorphisms of serotonin and dopamine transporters in mental disorders". The Journal of Medical Investigation 50 (1–2): 25–31. February 2003. PMID 12630565.
- ↑ "Delinquent peer group formation: evidence of a gene x environment correlation". The Journal of Genetic Psychology 169 (3): 227–44. September 2008. doi:10.3200/GNTP.169.3.227-244. PMID 18788325.
- ↑ Florida State University (2 October 2008). "Specific Gene Found In Adolescent Men With Delinquent Peers". ScienceDaily. https://www.sciencedaily.com/releases/2008/10/081001130004.htm.
- ↑ "Striatal dopamine transporter density in major depression". Psychopharmacology 144 (3): 282–5. June 1999. doi:10.1007/s002130051005. PMID 10435396.
- ↑ "Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood". Brain 137 (Pt 4): 1107–19. April 2014. doi:10.1093/brain/awu022. PMID 24613933.
- ↑ "Studies of the biogenic amine transporters 15. Identification of novel allosteric dopamine transporter ligands with nanomolar potency". The Journal of Pharmacology and Experimental Therapeutics 353 (3): 529–38. June 2015. doi:10.1124/jpet.114.222299. PMID 25788711.
- ↑ "Identification of a Novel Allosteric Modulator of the Human Dopamine Transporter". ACS Chem Neurosci 10 (8): 3718–3730. 2019. doi:10.1021/acschemneuro.9b00262. PMID 31184115.
- ↑ "Attenuation of dopamine transporter activity by alpha-synuclein". Neuroscience Letters 340 (3): 189–92. April 2003. doi:10.1016/S0304-3940(03)00097-1. PMID 12672538.
- ↑ "Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis". FASEB Journal 15 (6): 916–26. April 2001. doi:10.1096/fj.00-0334com. PMID 11292651.
- ↑ "Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1". Neuron 30 (1): 121–34. April 2001. doi:10.1016/S0896-6273(01)00267-7. PMID 11343649.
- ↑ "The multiple LIM domain-containing adaptor protein Hic-5 synaptically colocalizes and interacts with the dopamine transporter". The Journal of Neuroscience 22 (16): 7045–54. August 2002. doi:10.1523/JNEUROSCI.22-16-07045.2002. PMID 12177201.
- ↑ "Mutations at tyrosine 88, lysine 92 and tyrosine 470 of human dopamine transporter result in an attenuation of HIV-1 Tat-induced inhibition of dopamine transport". Journal of Neuroimmune Pharmacology 10 (1): 122–35. March 2015. doi:10.1007/s11481-015-9583-3. PMID 25604666.
- ↑ "Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions". Journal of Neuroimmune Pharmacology 8 (4): 975–87. September 2013. doi:10.1007/s11481-013-9464-6. PMID 23645138.
- ↑ "Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia". Molecular Neurobiology 44 (1): 102–10. August 2011. doi:10.1007/s12035-011-8195-z. PMID 21717292. https://zenodo.org/record/1232882.
External links
- Dopamine transporter-related Associations, Experiments, Publications and Clinical Trials
- Dopamine+Transporter at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: Q7K4Y6 (Drosophila melanogaster Sodium-dependent dopamine transporter) at the PDBe-KB.
 |