Chemistry:Cyclohexanedimethanol
| |
| Names | |
|---|---|
| IUPAC name
[4-(hydroxymethyl)cyclohexyl]methanol
| |
| Preferred IUPAC name
(cyclohexane-1,4-diyl)dimethanol | |
| Other names
1,4–Cyclohexanedimethanol; CHDM; 1,4-Bis(hydroxymethyl)cyclohexane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H16O2 | |
| Molar mass | 144.21 g/mol |
| Appearance | White waxy solid |
| Density | 1.02 g/ml |
| Melting point | 41 to 61 °C (106 to 142 °F; 314 to 334 K) |
| Boiling point | 284 to 288 °C (543 to 550 °F; 557 to 561 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
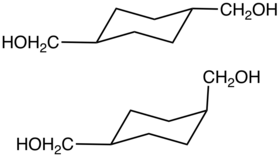
Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.
Applications
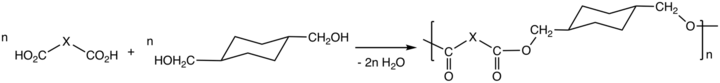
Via the process called polycondensation, CHDM is a precursor to polyesters. It is one of the most important comonomers for production of polyethylene terephthalate (PET), or polyethylene terephthalic ester (PETE), from which plastic bottles are made.[1][2] In addition it maybe spun to form carpet fibers.[3]
Thermoplastic polyesters containing CHDM exhibit enhanced strength, clarity, and solvent resistance. The properties of the polyesters vary from the high melting crystalline poly(1,4-cyclohexylenedimethylene terephthalate), PCT, to the non-crystalline copolyesters derived from both ethylene glycol and CHDM. The properties of these polyesters also is affected by the cis/trans ratio of the CHDM monomer.[4] CHDM reduces the degree of crystallinity of PET homopolymer, improving its processability. The copolymer tends to resist degradation, e.g. to acetaldehyde. The copolymer with PET is known as glycol-modified polyethylene terephthalate, PETG. PETG is used in many fields, including electronics, automobiles, barrier, and medical, etc.
CHDM may also be used to manufacture an epoxy diluent. Cyclohexanedimethanol is reacted with epichlorohydrin using a Lewis acid catalyst to form the halohydrin. The next step is to form the diglycidyl ether by washing the material with sodium hydroxide solution. This process is called, dehydrochlorination. The waste product formed is alkaline brine.[5] The key use for this diglycidyl ether is to reduce the viscosity of epoxy resins.[6]
Production
CHDM is produced by catalytic hydrogenation of dimethyl terephthalate (DMT). The reaction conducted in two steps beginning with the conversion of DMT to the diester dimethyl 1,4-cyclohexanedicarboxylate (DMCD):
- C6H4(CO2CH3)2 + 3 H2 → C6H10(CO2CH3)2
In the second step DMCD is further hydrogenated to CHDM:
- C6H10(CO2CH3)2 + 4 H2 → C6H10(CH2OH)2 + 2 CH3OH
A copper chromite catalyst is usually used industrially.[7] The cis/trans ratio of the CHDM is affected by the catalyst.[8]
Byproduct of this process are 4-methylcyclohexanemethanol (CH3C6H10CH2OH) and the monoester methyl 4-methyl-4-cyclohexanecarboxylate (CH3C6H10CO2CH3, CAS registry number 51181-40-9).[9] The leading producers in CHDM are Eastman Chemical in US and SK Chemicals in South Korea.
References
- ↑ S.R. Turner (2004). "Development of amorphous copolyesters based on 1,4- cyclohexane-dimethanol". Journal of Polymer Science Part A: Polymer Chemistry 42 (23): 5847–5852. doi:10.1002/pola.20460.
- ↑ S. Andjelic; D.D. Jamiolkowski; R. Bezwada (2007). "Mini-review The Polyoxaesters". Polymer International 56: 1063–1077. doi:10.1002/pi.2257.
- ↑ Hatton. "Nylon vs. Polyester Carpet Fibers: Comparison Guide" (in en-US). https://www.homedit.com/flooring/carpet/fibers/nylon-vs-polyester/.
- ↑ S. R. Turner; R.W. Seymour; T.W. Smith (2001). "Cyclohexanedimethanol Polyesters". Encyclopedia of Polymer Science and Technology. doi:10.1002/0471440264.pst257. ISBN 0471440264.
- ↑ Crivello, James V. (2006). "Design and synthesis of multifunctional glycidyl ethers that undergo frontal polymerization". Journal of Polymer Science Part A: Polymer Chemistry 44 (21): 6435–6448. doi:10.1002/pola.21761. ISSN 0887-624X. Bibcode: 2006JPoSA..44.6435C. https://doi.org/10.1002/pola.21761.
- ↑ Monte, Salvatore J. (1998), Pritchard, Geoffrey, ed., "Diluents and viscosity modifiers for epoxy resins" (in en), Plastics Additives: An A-Z reference, Polymer Science and Technology Series (Dordrecht: Springer Netherlands) 1: pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, https://doi.org/10.1007/978-94-011-5862-6_24, retrieved 2022-03-29
- ↑ S.R. Turner; Y. Li (2010). "Synthesis and Properties of Cyclic Diester Based Aliphatic Copolyesters". Journal of Polymer Science Part A: Polymer Chemistry 48 (10): 2162–2169. doi:10.1002/pola.23985.
- ↑ J. M. Thomas; R. Raja (2002). "The materials Chemistry of Inorganic Catalyst". Australian Journal of Chemistry 54: 551–560. doi:10.1071/CH01150.
- ↑ Peter Werle, Marcus Morawietz, Stefan Lundmark, Kent Sörensen, Esko Karvinen and Juha Lehtonen "Alcohols, Polyhydric" Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_305.pub2
External links
 |
