Chemistry:Cyclopentanepentone
From HandWiki
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Cyclopentane-1,2,3,4,5-pentone[1] | |||
| Other names
Leuconic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
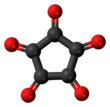
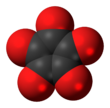
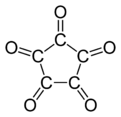
| C5O5 | |||
| Molar mass | 140.050 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
Cyclopentanepentone, also known as leuconic acid, is a hypothetical organic compound with formula C5O5, the fivefold ketone of cyclopentane. It would be an oxide of carbon (an oxocarbon), indeed a pentamer of carbon monoxide.
As of 2000, the compound had yet to be synthesized in bulk, but there have been reports of trace synthesis.[2][3][4]
Related compounds
Cyclopentanepentone can be viewed as the neutral counterpart of the croconate anion C5O2−5.
The compound referred to in the literature and trade as "cyclopentanepentone pentahydrate" (C5O5·5H2O) is probably decahydroxycyclopentane (C5(OH)10).[3][5]
References
- ↑ "CID 12305030 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=12305030&loc=ec_rcs.
- ↑ Rubin, M. B.; Gleiter, R. (2000). "The Chemistry of Vicinal Polycarbonyl Compounds". Chemical Reviews 100 (3): 1121–64. doi:10.1021/cr960079j. PMID 11749259.
- ↑ Jump up to: 3.0 3.1 Seitz, G.; Imming, P. (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- ↑ Schröder, D.; Schwarz, H.; Dua, S.; Blanksby, S. J.; Bowie, J. H. (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry 188 (1–2): 17–25. doi:10.1016/S1387-3806(98)14208-2. Bibcode: 1999IJMSp.188...17S.
- ↑ Person, W. B.; Williams, D. G. (1957). "Infrared Spectra and the Structure of Leuconic Acid and Triquinoyl". Journal of Physical Chemistry 61 (7): 1017–1018. doi:10.1021/j150553a047.
See also
 |