Chemistry:Diethyl carbonate
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Diethyl carbonate | |
| Other names
Carbonic ether; Ethyl carbonate, di-; Eufin[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2366 |
| |
| |
| Properties | |
| C5H10O3 | |
| Molar mass | 118.132 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.975 g/cm3 |
| Melting point | −43 °C (−45 °F; 230 K) [2] |
| Boiling point | 125.9 °C (258.6 °F; 399.0 K) [2] |
| Insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
| Flash point | 33 °C (91 °F; 306 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
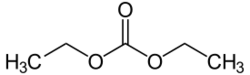
Diethyl carbonate (sometimes abbreviated DEC) is an ester of carbonic acid and ethanol with the formula OC(OCH2CH3)2. At room temperature (25 °C) diethyl carbonate is a colorless liquid with a low flash point.
Diethyl carbonate is used as a solvent such as in erythromycin intramuscular injections.[3][4][5] It can be used as a component of electrolytes in lithium batteries. It has been proposed as a fuel additive to support cleaner diesel fuel combustion because its high boiling point might reduce blended fuels' volatility, minimizing vapor buildup in warm weather that can block fuel lines.[6] As a fuel additive, it can reduce emissions such as volatile organic compounds, CO2, and particulates.[7]
Production
It can be made by reacting phosgene with ethanol, producing hydrogen chloride as a byproduct. Because chloroform can react with oxygen to form phosgene, chloroform can be stabilized for storage by adding 1 part (by mass) of ethanol to 100 parts (by mass) of chloroform, so that any phosgene that forms is converted into diethyl carbonate.
It can also be made by the alcoholysis of urea with ethanol. This reaction requires a heterogeneous catalysis that can act both as a Lewis acid and a base, such as various metal oxides. The reaction proceeds via the formation of the intermediary ethyl carbamate.[7]
It can also be synthesized directly from carbon dioxide and ethanol using various methods, and via oxidative carbonylation with carbon monoxide. Another method is transesterification from dimethyl carbonate. Yet another method is from the reaction of ethyl nitrite and carbon monoxide, where the ethyl nitrite can be made from nitric oxide and ethanol. This method requires a catalyst such as palladium.[7]
Use in biological research
0.01% v/v DEC solutions can be used as a relatively gentle cold sterilizing reagent for laboratory chromatography resins.[8]
See also
References
- ↑ "DIETHYL CARBONATE". http://chemicalland21.com/industrialchem/solalc/DIETHYL%20CARBONATE.htm. Retrieved 2010-02-01.
- ↑ 2.0 2.1 William M. Haynes, ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 15-15. ISBN 978-1498754293.
- ↑ Anderson, Robert C.; Harris, Paul N.; Chen, K. K. (1955). "Further toxicological studies with ilotycin® (Erythromycin, Lilly)" (in en). Journal of the American Pharmaceutical Association 44 (4): 199–204. doi:10.1002/jps.3030440404. ISSN 1930-2304. PMID 14367139. https://onlinelibrary.wiley.com/doi/abs/10.1002/jps.3030440404.
- ↑ Sciavolino, Frank C. & James R. Hauske, "9-Dihydro-11,12-ketal derivatives of erythromycin A and epi-erythromycin A", US patent 4382086, published 1983-05-03, issued 1982-03-01
- ↑ Hauske, James R., "3",4"-Oxyallylene erythromycin and oleandomycin, composition and method of use", US patent 4363803, published 1982-12-14, issued 1982-03-01
- ↑ Walter, K. Scientists Discover Method for Cleaner Fossil Fuel. MR&D Magazine. 09/18/2017 - 3:16pm
- ↑ 7.0 7.1 7.2 Shukla, Kartikeya; Srivastava, Vimal Chandra (2016). "Diethyl carbonate: critical review of synthesis routes, catalysts used and engineering aspects". RSC Advances 6 (39): 32624–32645. doi:10.1039/c6ra02518h. Bibcode: 2016RSCAd...632624S. https://pubs.rsc.org/en/content/pdf/article/2016/ra/c6ra02518h. Retrieved Aug 3, 2021.
- ↑ Rad, Bio-. "Bio-Gel A Gels - Instruction Manual". https://www.bio-rad.com/webroot/web/pdf/lsr/literature/4006139.pdf.
 |

