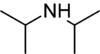
Chemistry:Diisopropylamine
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(Propan-2-yl)propan-2-amine | |
| Other names
Di(propan-2-yl)amine
N-Isopropylpropan-2-amine (Diisopropyl)amine (The name diisopropylamine is deprecated.) | |
| Identifiers | |
3D model (JSmol)
|
|
| 605284 | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1158 |
| |
| |
| Properties | |
| C6H15N | |
| Molar mass | 101.193 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 0.722 g mL−1 |
| Melting point | −61.00 °C; −77.80 °F; 212.15 K |
| Boiling point | 83 to 85 °C; 181 to 185 °F; 356 to 358 K |
| miscible[1] | |
| Vapor pressure | 9.3 kPa (at 20°C)[2] |
| Acidity (pKa) | 11.07 (in water) (conjugate acid) |
| Basicity (pKb) | 3.43[2] |
Refractive index (nD)
|
1.392–1.393 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−173.6 to −168.4 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4.3363 to −4.3313 MJ mol−1 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | DANGER |
| H225, H302, H314, H332 | |
| P210, P280, P305+351+338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | −17 °C (1 °F; 256 K) |
| 315 °C (599 °F; 588 K) | |
| Explosive limits | 1.1–7.1%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
LC50 (median concentration)
|
1140 ppm (rat, 2 hr) 1000 ppm (mouse, 2 hr)[3] |
LCLo (lowest published)
|
2207 ppm (rabbit, 2.5 hr) 2207 ppm (guinea pig, 80 min) 2207 ppm (cat, 72 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 5 ppm (20 mg/m3) [skin][1] |
REL (Recommended)
|
TWA 5 ppm (20 mg/m3) [skin][1] |
IDLH (Immediate danger)
|
200 ppm[1] |
| Related compounds | |
Related amines
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent.
Reactions and use
Diisopropylamine is a common amine nucleophile in organic synthesis.[4] Because it is bulky, it is a more selective nucleophile than other similar amines, such as dimethylamine.[5]
It reacts with organolithium reagents to give lithium diisopropylamide (LDA). LDA is a strong, non-nucleophilic base[6]
The main commercial applications of diisopropylamine is as a precursor to the herbicide, diallate and triallate as well as certain sulfenamides used in the vulcanization of rubber.[7]
It is also used to prepare N,N-Diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate.[8]
The bromide salt of diisopropylamine, diisopropylammonium bromide, is a room-temperature organic ferroelectric material.[9]
Preparation
Diisopropylamine, which is commercially available, may be prepared by the reductive amination of acetone with ammonia using a modified copper oxide, generally copper chromite, as a catalyst:[10][11]
- NH
3 + 2 (CH
3)
2CO + 2 H
2 → C
6H
15N + 2 H
2O
Diisopropylamine can be dried by distillation from potassium hydroxide (KOH) or drying over sodium wire.[12](p186)
Toxicity
Diisopropylamine causes burns by all exposure routes. Inhalation of high concentrations of its vapor may cause symptoms like headache, dizziness, tiredness, nausea and vomiting.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 NIOSH Pocket Guide to Chemical Hazards. "#0217". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0217.html.
- ↑ 2.0 2.1 Template:Pubchem
- ↑ 3.0 3.1 "Diisopropylamine". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/108189.html.
- ↑ John E. McMurry, Jack Melton (1977). "Conversion of Nitro to Carbonyl by Ozonolysis of Nitronates: 2,5-Heptanedione". Organic Syntheses 56: 36. doi:10.15227/orgsyn.056.0036.
- ↑ Denmark, Scott; Ryabchuk, Pavel; Min Chi, Hyung; Matviitsuk, Anastassia (2019). "Preparation of a Diisopropylselenophosphoramide Catalyst and its Use in Enantioselective Sulfenoetherification". Organic Syntheses 96: 400–417. doi:10.15227/orgsyn.096.0400. PMID 34526731.
- ↑ George M. Rubottom, John M. Gruber, Henrik D. Juve, Jr, , Dan A. Charleson (1986). "α-Hydroxy Ketones from the Oxidation of Enol Silyl Ethers with m-Chloroperbenzoic Acid: 6-Hydroxy- 3,5,5-trimethyl-2-cyclohexen-1-one". Organic Syntheses 64: 118. doi:10.15227/orgsyn.064.0118.
- ↑ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000-06-15). "Amines, Aliphatic". Amines, Aliphatic. Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 978-3527303854.
- ↑ Hünig, Siegfried; Kiessel, Max (1958-04-01). "Spezifische Protonenacceptoren als Hilfsbasen bei Alkylierungs- und Dehydrohalogenierungsreaktionen" (in de). Chemische Berichte (Wiley-VCH) 91 (2): 380-392. doi:10.1002/cber.19580910223. ISSN 0009-2940. OCLC 889715844.
- ↑ Fu, Da-Wei; Cai, Hong-Ling; Liu, Yuanming; Ye, Qiong; Zhang, Wen et al. (2013-01-25). "Diisopropylammonium Bromide Is a High-Temperature Molecular Ferroelectric Crystal" (in en). Science 339 (6118): 425-428. doi:10.1126/science.1229675. ISSN 0036-8075. OCLC 1644869. PMID 23349285. Bibcode: 2013Sci...339..425F.
- ↑ Löffler, Karl (1910-04-01). "Über eine neue Bildungsweise primärer und sekundärer Amine aus Ketonen" (in de). Berichte der Deutschen Chemischen Gesellschaft 43 (2): 2031–2035. doi:10.1002/cber.191004302145. ISSN 0365-9496. OCLC 219854722. https://zenodo.org/record/1426399.
- ↑ Willard Bull, "One-step process for preparing diisopropylamine", US patent 2686811
- ↑ Armarego, W. L. F.; Perrin, D. D. (1996-10-16). Purification of Laboratory Chemicals (4th ed.). Butterworth-Heinemann. ISBN 978-0750628396. OCLC 762966259.
 |


