Chemistry:Dinitro-ortho-cresol
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-3,5-dinitrophenol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C7H6N2O5 | |
| Molar mass | 198.134 g·mol−1 |
| Appearance | Yellow solid[1] |
| Odor | Odorless[1] |
| Density | 1.58 g/cm3 |
| Melting point | 86.5 °C (187.7 °F; 359.6 K) |
| Boiling point | 312 °C (594 °F; 585 K) |
| 0.01% (20°C)[1] | |
| Vapor pressure | 0.00005 mmHg (20°C)[1] |
| Hazards | |
| Flash point | noncombustible [1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
7 mg/kg (oral, rat) 50 mg/kg (oral, cat) 21 mg/kg (oral, mouse) 24.6 mg/kg (oral, rabbit) 24.6 mg/kg (oral, guinea pig) 31 mg/kg (oral, rat)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.2 mg/m3 [skin][1] |
REL (Recommended)
|
TWA 0.2 mg/m3 [skin][1] |
IDLH (Immediate danger)
|
5 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
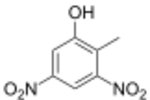
Dinitro-ortho-cresol (DNOC) is an organic compound with the structural formula CH3C6H2(NO2)2OH. It is a yellow solid that is only slightly soluble in water. It is extremely toxic to humans and was previously used as a herbicide and insecticide.
Preparation
This compound is prepared by disulfonation of o-cresol. The resulting disulfonate is then treated with nitric acid to give DNOC. A variety of related derivatives are known including those where the methyl group is replaced by sec-butyl (dinoseb), tert-butyl (dinoterb), and 1-methylheptyl (dinocap). These are prepared by the direct nitration of the alkyphenols.[3]
Applications and safety
DNOC is an uncoupler, which means that it interferes with adenosine triphosphate (ATP) production,[4][5] making it extremely toxic to humans.[6]
DNOC was one of the earliest pesticides developed, being used as an insecticide since the 1890s and a herbicide since the 1930s.[7] It was banned for use as a pesticide in the United States in 1991.[6]
Symptoms of dinitro-ortho-cresol poisoning, due to ingestion or other forms of exposure, include confusion, headache, shortness of breath, and sweating.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 NIOSH Pocket Guide to Chemical Hazards. "#0234". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0234.html.
- ↑ "Dinitro-o-cresol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. https://www.cdc.gov/niosh/idlh/534521.html. Retrieved 17 March 2015.
- ↑ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
- ↑ Parker, V. H.; Barnes, J. M.; Denz, F. A. (1951). "Some Observations on the Toxic Properties of 3:5-Dinitro-Ortho-Cresol". Occupational and Environmental Medicine 8 (4): 226. doi:10.1136/oem.8.4.226. PMID 14878957.
- ↑ Harvey, DG; Bidstrup, PL; Bonnell, JA (1951). "Poisoning by dinitro-ortho-cresol; some observations on the effects of dinitro-ortho-cresol administered by mouth to human volunteers". British Medical Journal 2 (4722): 13–6. doi:10.1136/bmj.2.4722.13. PMID 14839311.
- ↑ 6.0 6.1 "4,6-DINITRO-o-CRESOL (DNOC) (including salts)". https://www.epa.gov/sites/default/files/2016-09/documents/4-6-dinitro-o-cresol.pdf.
- ↑ Biegaǹska, Jolanta (1 February 2005). "Neutralization of 4,6-Dinitro- o -cresol Waste Pesticide by Means of Detonative Combustion". Environmental Science & Technology 39 (4): 1190–1196. doi:10.1021/es035327p.
- ↑ "Chemical poisoning -- Dinitrocresol Symptoms, Diagnosis, Treatments and Causes - RightDiagnosis.com". http://www.rightdiagnosis.com/c/chemical_poisoning_dinitrocresol/intro.htm.
External links
 |

