Chemistry:Diphenylketene

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Diphenylethen-1-one | |
| Other names
Diphenylethenone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H10O | |
| Molar mass | 194.233 g·mol−1 |
| Appearance | Red-orange oil |
| Melting point | 8 to 9 °C (46 to 48 °F; 281 to 282 K) |
| Boiling point | 118 to 120 at 1mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.[1]
History
Diphenyl ketene was first isolated by Hermann Staudinger in 1905 and identified as the first example of the exceptionally reactive class of ketenes[2] with the general formula R1R2C=C=O (R1=R2=phenyl group).[3]
Preparation
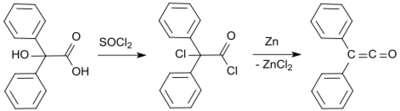
The first synthesis by H. Staudinger was based on 2-chlorodiphenylacetyl chloride (prepared from benzilic acid and thionyl chloride[4]) from which two chlorine atoms are cleaved with zinc in a dehalogenation reaction:[2]
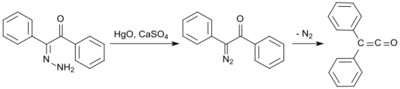
An early synthesis uses benzilmonohydrazone (from Diphenylethanedione and hydrazine hydrate[5]), which is oxidized with mercury(II)oxide and calcium sulfate to form mono-diazoketone, and is then converted into the diphenylketene at 100 °C under nitrogen elimination in 58% yield:[6]
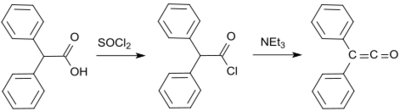
A further early diphenylketene synthesis originates from Eduard Wedekind, who had already obtained diphenyl ketene in 1901 by the dehydrohalogenation of diphenylacetyl chloride with triethylamine, without isolation and characterization though.[7] This variant was also described in 1911 by H. Staudinger.[8]
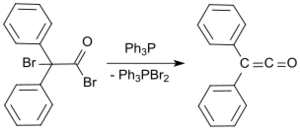
A standard laboratory protocol is based on the Staudinger method and yields diphenyl ketene as an orange oil in yields of 53 to 57%.[9] In a more recent process, 2-bromo-2,2-diphenylacetyl bromide is reacted with triphenylphosphine to give diphenyl ketene in yields up to 81%.[10]
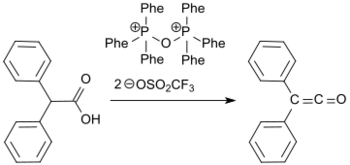
Recently, a synthesis of diphenyl ketene from diphenylacetic acid and the Hendrickson reagent (triphenylphosphonium anhydride-trifluoromethanesulfonate)[11] with water elimination in 72% yield has been reported.[12]
Properties
Diphenyl ketene is at room temperature an orange-colored to red oil (with the color of concentrated potassium dichromate solution[2]) which is miscible with nonpolar organic solvents (such as diethyl ether, acetone, benzene, tetrahydrofuran, chloroform)[13] and solidifies in the cold forming yellow crystals.[2] The compound is easily oxidized by air but can be stored in tightly closed containers at 0 °C for several weeks without decomposition[9] or in a nitrogen atmosphere with the addition of a small amount of hydroquinone as a polymerization inhibitor.[6]
Reactivity
Diphenylketene can undergo attack from a host of nucleophiles, including alcohols, amines, and enolates with fairly slow rates. These rates can be increased in the presence of catalysts. At present the mechanism of attack is unknown, but work is underway to determine the exact mechanism.
The high reactivity of the diphenyl ketene is also evident in the formation of three dimers:[14]
- the cyclic diketone 2,2,4,4-tetraphenylcyclobutane-1,3-dione (I) by heating with quinoline
- the β-lactone 4-(diphenylmethylene)-3,3-diphenyloxetan-2-one (II) by heating with sodium methoxide and
- the tetraline derivative 2,2,4-triphenylnaphthalene-1,3-(2H,4H)-dione (III) by heating with benzoyl chloride
and oligomers produced therefrom.
Application
Ketenes (of the general formula R1R2C=C=O) have many parallels to isocyanates (of the general formula R-N=C=O) in their constitution as well as in their reactivity.
Diphenyl ketene reacts with water in an addition reaction to form diphenylacetic acid, with ethanol to diphenyl acetic ethyl ester or with ammonia to the corresponding amide.[2] Carboxylic acids produce mixed anhydrides of diphenylacetic acid, which can be used to activate protected amino acids for peptide linkage.
- [math]\displaystyle{ \ce{(Phenyl)2C=C=O -\gt [{}\atop\text{Z-Leu}] (Phenyl)2CO-O-CO-{}}\text{Z-Leu }\ce{-\gt [{}\atop\ce{H-Phe-OEt}]}\text{ Z-Leu}\ce{-Phe-OEt} }[/math]
The protected dipeptide Z-Leu-Phe-OEt (N-benzyloxycarbonyl-L-leucyl-L-phenylalanine ethyl ester) is thus obtained in 59% yield via the activation of Z-leucine with diphenyl ketene and subsequent reaction with phenylalanine ethyl ester.[15]
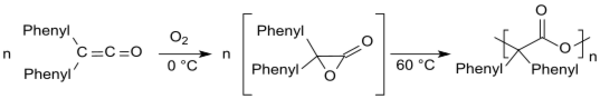
Diphenyl ketene is prone to autoxidation, in which the corresponding polyester is formed at temperatures above 60 °C via an intermediate diphenyl acetolactone.[16]
In a Wittig reaction, allenes can be prepared from diphenyl ketene.[17]
With triphenylphosphine diphenylmethylene and diphenyl ketene, at e. g. 140 °C and under pressure tetraphenyl allenes are formed in 70% yield.[18]
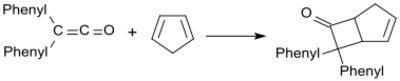
The synthetically most interesting reactions of diphenyl ketene are [2+2]cycloadditions, e.g. the reaction with cyclopentadiene yielding a Diels-Alder adduct.[19]
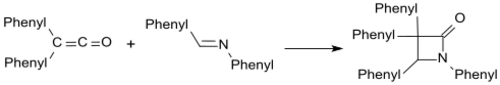
Imines such as benzalaniline form β-lactams with diphenyl ketene.
With carbonyl compounds β-lactones are formed analogously.[19]
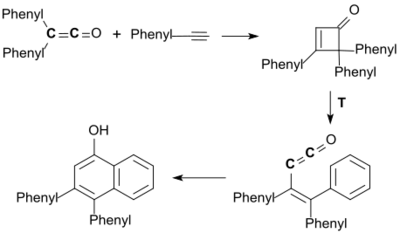
The [2+2]cycloaddition of diphenyl ketene with phenylacetylene leads first to a cyclobutenone which thermally aromatizes to a phenyl vinyl ketene and cyclizes in a [4+2]cycloaddition to 3,4-diphenyl-1-naphthol in 81% yield.[20]
From this so-called Smith-Hoehn reaction a general synthesis method for substituted phenols and quinones has been developed.[3]
References
- ↑ Ulrich, H. (1967), Cycloaddition Reactions of Heterocumulenes, New York: Academic Press, p. 374
- ↑ 2.0 2.1 2.2 2.3 2.4 Staudinger, H. (1905). "Ketene, eine neue Körperklasse" (in German). Ber. Dtsch. Chem. Ges. 38 (2): 1735–1739. doi:10.1002/cber.19050380283. https://zenodo.org/record/1426156.
- ↑ 3.0 3.1 Tidwell, T.T. (2005), "The first century of ketenes (1905–2005): The birth of a versatile family of reactive intermediates", Angew. Chem. 44 (36): 5778–5785, doi:10.1002/anie.200500098, PMID 16149113
- ↑ King, F.E.; Holmes, D. (1947), "Synthetic mydriatics. Diphenylchloroacetyl chloride as a reagent for the preparation of benzylic esters of tertiary amino-alcohols", J. Chem. Soc.: 164–168, doi:10.1039/JR9470000164, PMID 20238643
- ↑ Curtius, T.; Thun, K. (1891), "Einwirkung von Hydrazinhydrat auf Monoketone und Orthodiketone", J. Prakt. Chem. 44 (2): 161–186, doi:10.1002/prac.18910440121, https://zenodo.org/record/1427962
- ↑ 6.0 6.1 Smith, L.I.; Hoehn, H.H. (1940). "Diphenylketene [Ketene, diphenyl-"]. Organic Syntheses 20: 47. doi:10.15227/orgsyn.020.0047. http://www.orgsyn.org/demo.aspx?prep=CV3P0356.; Collective Volume, 3, pp. 356
- ↑ Wedekind, E. (1901), "Ueber die Gewinnung von Säureanhydriden mit Hülfe von tertiären Aminen", Ber. Dtsch. Chem. Ges. 34 (2): 2070–2077, doi:10.1002/cber.190103402122, https://zenodo.org/record/1426004
- ↑ Staudinger, H. (1911), "Über Ketene.XIX. Über Bildung und Darstellung des Diphenylketens", Ber. Dtsch. Chem. Ges. 44 (2): 1619–1623, doi:10.1002/cber.19110440258, https://zenodo.org/record/1426443
- ↑ 9.0 9.1 Taylor, E.C.; McKillop, A; Hawks, G.H. (1972). "Diphenylketene [Ethenone, diphenyl-"]. Organic Syntheses 52: 36. doi:10.15227/orgsyn.052.0036. http://www.orgsyn.org/demo.aspx?prep=CV6P0549.; Collective Volume, 6, pp. 549
- ↑ Darling, S.D.; Kidwell, R.L. (1968), "Diphenylketene. Triphenylphosphine dehalogenation of .alpha.-bromodiphenylacetyl bromide", J. Org. Chem. 33 (10): 3974–3975, doi:10.1021/jo01274a074
- ↑ McCauley, J.I. (2012), "Hendrickson reagent (triphenylphosphonium anhydride trifluormethane sulfonate", Synlett 23 (20): 2999–3000, doi:10.1055/s-0032-1317486
- ↑ Moussa, Z. (2012), "The Hendrickson 'POP' reagent and analogues thereof: synthesis, structure, and application in organic synthesis", Arkivoc 2012 (1): 432–490, doi:10.3998/ark.5550190.0013.111
- ↑ Leahy, J.W. (2001). Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd421. ISBN 0471936235.
- ↑ Das, H.; Kooyman, E. C. (1965). "Oligomers of diphenylketene". Recueil des Travaux Chimiques des Pays-Bas 84 (8): 965–978. doi:10.1002/recl.19650840802.
- ↑ Losse, G.; Demuth, E. (1961), "Diphenylketen als Reagens zur Knüpfung von Peptidbindungen" (in German), Ber. Dtsch. Chem. Ges. 94 (7): 1762–1766, doi:10.1002/cber.19610940713
- ↑ Staudinger, H.; Dyckerhoff, K.; Klever, H.W.; Ruzicka, L. (1925), "Über Autoxidation organischer Verbindungen. IV.: Über Autoxidation der Ketene" (in German), Ber. Dtsch. Chem. Ges. 58 (6): 1079–1087, doi:10.1002/cber.19250580618
- ↑ Wittig, G.; Haag, A. (1963), "Über Phosphin-alkylene als olefinbildende Reagenzien, VIII. Allelderivate aus Ketenen" (in German), Ber. Dtsch. Chem. Ges. 96 (6): 1535–1543, doi:10.1002/cber.19630960609
- ↑ Lüscher, G. (1922). Beitrag zur Konstitution der aliphatischen Diazokörper und Hydrazone. Neue organische Phosphorverbindungen (PDF) (Doctoral Thesis) (in German). Eidgenössische Technische Hochschule Zurich. doi:10.3929/ethz-a-000096667. hdl:20.500.11850/134328.CS1 maint: unrecognized language (link)
- ↑ 19.0 19.1 Staudinger, H. (1907), "Zur Kenntnis der Ketene. Diphenylketen" (in German), Liebigs Ann. Chem. 356 (1–2): 51–123, doi:10.1002/jlac.19073560106, https://zenodo.org/record/1427571
- ↑ Smith, L.I.; Hoehn, H.H. (1939), "The reaction of diphenylketene and phenylacetylene", J. Am. Chem. Soc. 61 (10): 2619–2624, doi:10.1021/ja01265a015
 |