Chemistry:Disodium octaborate
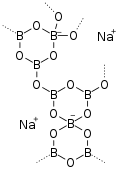 Repeating asymmetrical unit in α-Na2B8O13
| |
| Names | |
|---|---|
| IUPAC name
Disodium;(9,11-dioxido-5-oxoboranyloxy-2,4,6,8,10,12,13-heptaoxa-1,3,5,7,9,11-hexaborabicyclo[5.5.1]tridecan-3-yl)oxy-oxoborane
| |
| Other names
Sodium octaborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Na 2B 8O 13 | |
| Molar mass | 340.45 g·mol−1 |
| Appearance | Colorless crystals |
| 9.5 g/(100 g)[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na 2B 8O 13 · 4H2O | |
| Molar mass | 412.5270 g/mol |
| Appearance | white powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Disodium octaborate is a borate of sodium, a chemical compound of sodium, boron, and oxygen — a salt with elemental formula Na
2B
8O
13 or (Na+
)
2[B
8O
13]2−, also written as Na
2O · 4B
2O
3. It is a colorless crystalline solid, soluble in water.
Disodium octaborate is traded either as a liquid concentrate, or as the tetrahydrate Na
2B
8O
13 · 4H2O, a white odorless powder. It is used as an insecticide,[3][4] and as a fungicide and algicide, and as a fire retardant.,[5][6] and as a boron micronutrient additive in fertilizers.[1] Trade names include Bora-Care, Borathor, Termite Prufe, Board Defense, Polybor,[6] Tim-bor,[7] Mr Dotty 008, and Can-Bor.
Preparation
The anhydrous form can be crystallized from a molten mixture of sodium oxide Na
2O and boric oxide B
2O
3.[8]
Properties
Solubility
The salt dissolves in water to form forms viscous supersaturated solutions at elevated temperatures. Solubility of the tetrahydrate is 21.9% (wt) at 30 °C (303 K).[9]
Structure
The anhydrous salt exists in two stable crystalline forms, α and β.[10][11]
The α form has monoclinic crystal structure, with the P21/a space group. The unit cell parameters at 273 K are: a = 650.7 pm, b = 1779 pm, c = 837.7 pm, β = 96.6 °, Z = 4. The structure contains two interlocking boron-oxygen frameworks, each of them consisting of alternating single and double rings composed of two triangles and a tetrahedron, the so called triborate and pentaborate groups. The two frameworks are connected by two (non-equivalent) sodium atoms, each surrounded by 8 oxygens, comprising finite chains of four NaO
8 polyhedra with shared edges. The thermal expansion is sharply anisotropic, including negative thermal expansion. The thermal expansion tensor in 273–1000 K in function of absolute temperature T has α11 = 55–0.042T, α22 = 11, α33 = -15 + 0.032T (×10–6) K−1, μ = (c^α33) = 42°.[10]
The β form has monoclinic crystal structure, with the P21/c space group. The unit cell parameters are a = 1173.1 pm, b = 788.0 pm, c = 1041.0 pm, β = 99.883 °, Z = 4. The structure consists of two infinite, independent, and interleaved boron–oxygen networks containing a complex borate anion [B
8O
13]2− formed by six BO
3 triangles (Δ) and two BO
4 tetrahedra (T), which can be viewed as a B
5O
10 group linked to a B
3O
7 group. This fundamental building block is identical to that of the α form and of silver octaborate Ag
2B
8O
13, with some subtle differences.[11]
Uses
Dilute solutions of disodium octaborate are sprayed on wood surfaces to kill termites, powder post beetles, carpenter ants, fungi and algae. The tetrahydrate is also available as pellets for embedding in structural wood. Compared to other chemicals used for these purposes, it has the advantages of lack of odor, permanent effect, and low toxicity to humans and pets.[4]
The compound was also shown to significantly reduce dust mite populations in the home when applied as a dilute solution to carpets and upholstery together with regular vacuum cleaning.[12]
Disodium octaborate, applied to the soil or foliar spray, has been shown to inhibit pests of crops such as tomato and pistachio, with no observed detrimental effects to the plants.[13][14]
Safety
Disodium octaborate is neither flammable, nor combustible or explosive and has low acute oral and dermal toxicity.[3] The oral 50% lethal doses (LD50) are 5.3 g/kg for guinea pig, 2g/kg for rats.[4] However, it is classified as "reproductive toxicity category 1B (presumed human reproductive toxicant)" under the EU Classification, Labelling and Packaging Regulation (CLP Regulation). The CLP hazard code and statement are "H360FD: May damage fertility. May damage the unborn child."
On 22 February 2018, the Swedish Chemicals Agency (KEMI) submitted a proposal to the European Chemicals Agency (ECHA) to list disodium borate as a Substance of Very High Concern (SVHC) under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation.[15]
References
- ↑ 1.0 1.1 "Boron sodium oxide (B8Na2O13)". Substance page at the PubChem website. Accessed on 2022-06-28.
- ↑ H. W. Scherer et al (2008): "Fertilizers". Chapter of Ullmann's Encyclopedia of Industrial Chemistry, 7th ed.
- ↑ 3.0 3.1 Su, NAN-YAO; Scheffrahn, Rudolf H. (1998). "A review of subterranean termite control practices and prospects for integrated pest management programmes". Integrated Pest Management Reviews 3: 1–13. doi:10.1023/A:1009684821954.
- ↑ 4.0 4.1 4.2 "Disodium octaborate". Substance page at the National Institutes of Health - National Library of Medicine ChemDplus website. Accessed on 2022-06-28.
- ↑ B.J Brotherton Boron: Inorganic Chemistry Encyclopedia of Inorganic Chemistry (1994) Ed. R. Bruce King, John Wiley & Sons ISBN 0-471-93620-0
- ↑ 6.0 6.1 "Polybor". Product data page at the U. S. Borax company website. Accessed on 2022-06-27.
- ↑ "[1] ". Product data page at the U. S. Borax company website. Accessed on 2022-06-27.
- ↑ C. J. Leedecke and C. G. Bergeron (1977): "Crystallisation of Na2B8O13 in Selected Na2O-B2O3 Melts". Physics and Chemistry of Glasses (UK), volume 18, issue 6, pages 116-120.
- ↑ M. Briggs (2001): "Boron Oxides, Boric Acid, and Borates". Chapter of the Kirk-Othmer Encyclopedia of Chemical Technology. Wiley.
- ↑ 10.0 10.1 Bubnova, R. S.; Shepelev, Ju. F.; Sennova, N. A.; Filatov, S. K. (2002). "Thermal behaviour of the rigid boron-oxygen groups in the α-Na2B8O13 crystal structure". Zeitschrift für Kristallographie - Crystalline Materials 217 (9): 444–450. doi:10.1524/zkri.217.9.444.22881. Bibcode: 2002ZK....217..444B.
- ↑ 11.0 11.1 Penin, N.; Touboul, M.; Nowogrocki, G. (2002). "Crystal Structure of a New Form of Sodium Octoborate β-Na2B8O13". Journal of Solid State Chemistry 168 (1): 316–321. doi:10.1006/jssc.2002.9704. Bibcode: 2002JSSCh.168..316P.
- ↑ Codina, R.; Lockey, R. F.; Diwadkar, R.; Mobly, L. L.; Godfrey, S. (2003). "Disodium octaborate tetrahydrate (DOT) application and vacuum cleaning, a combined strategy to control house dust mites". Allergy 58 (4): 318–324. doi:10.1034/j.1398-9995.2003.00100.x. PMID 12708980.
- ↑ H. Kavak, A. L. Tuna, and H. S. Civelek (2017): "Effects of Tarımbor (Na2B8O13.4(H2O)) fertiliser against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and biochemistry and physiology of tomato plants under greenhouse conditions." Ege Üniversitesi Ziraat Fakültesi Dergisi, volume 54, issue 2, pages 157-165 (in Turkish).
- ↑ Açar, İzzet; Doran, İlhan; Aslan, Nevzat; Doğruer Kalkanci, Nilgün (2016). "Boron affects the yield and quality of nonirrigated pistachio (Pistacia vera L.) trees". Turkish Journal of Agriculture and Forestry 40: 664–670. doi:10.3906/tar-1511-80. https://dergipark.org.tr/tr/pub/tbtkagriculture/issue/34745/384233.
- ↑ "Proposal for Identification of a Substance of Very High Concern on the Basis of the Criteria Set Out in REACH Article 57", Swedish Chemicals Agency, 22 February 2018.
 |
