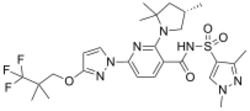
Chemistry:Elexacaftor
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Trikafta and Kaftrio (with ivacaftor and tezacaftor) |
| Other names | VX-445 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619061 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H34F3N7O4S |
| Molar mass | 597.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Elexacaftor is a medication that acts as cystic fibrosis transmembrane conductance regulator (CFTR) corrector.[1]
It is available in a single pill with ivacaftor and tezacaftor; the fixed-dose combination, elexacaftor/tezacaftor/ivacaftor (brand name Trikafta), is used to treat people with cystic fibrosis who are homozygous for the f508del mutation.[1][2] This combination was approved for medical use in the United States in 2019.[1][3][4]
The fixed-dose combination elexacaftor/tezacaftor/ivacaftor (Kaftrio) was approved for medical use in the European Union in August 2020, for the treatment of cystic fibrosis.[5][6]
References
- ↑ 1.0 1.1 1.2 "Trikafta (elexacaftor, ivacaftor and tezacaftor) Patient Information". October 23, 2019. https://www.drugs.com/trikafta.html.
- ↑ "Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy". The Journal of Pediatric Pharmacology and Therapeutics 25 (3): 192–197. 2020. doi:10.5863/1551-6776-25.3.192. PMID 32265602.
- ↑ "FDA approves new breakthrough therapy for cystic fibrosis". U.S. Food and Drug Administration (FDA) (Press release). October 21, 2019. Archived from the original on November 13, 2019. Retrieved November 13, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Trials Snapshots: Trikafta". October 31, 2019. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-trikafta.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Kaftrio EPAR". 23 June 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/kaftrio.
- ↑ "New medicine for cystic fibrosis patients". European Medicines Agency (EMA) (Press release). 26 June 2020. Retrieved 26 June 2020.
External links
- "Elexacaftor". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/elexacaftor.
 |

