Chemistry:Tezacaftor
 | |
| Clinical data | |
|---|---|
| Trade names | Symdeko (with ivacaftor) |
| Other names | VX-661 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
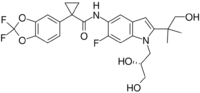
| Formula | C26H27F3N2O6 |
| Molar mass | 520.505 g·mol−1 |
| |
Tezacaftor is a drug used for the treatment of cystic fibrosis (CF) in people six years and older, who have a F508del mutation, the most common type of mutation in the CFTR gene.[2][3] It is sold as a fixed-dose combination with ivacaftor under the brand name Symdeko.[4][5][6] It was approved by the U.S. FDA in 2018.[2] The combination of elexacaftor, tezacaftor, and ivacaftor is being sold as Trikafta.[7]
In 2019, the U.S. Food and Drug Administration (FDA) approved a combination of elexacaftor, tezacaftor, and ivacaftor.[8]
Mechanism of action
Tezacaftor acts as a corrector to help the folding and presentation of the CFTR protein to the cell surface, which improves its function for individuals with a F508del mutation.[2][9][10]
Clinical trials
The EVOLVE and EXPAND study findings were published in 2017.[11]
EVOLVE trial
The EVOLVE trial analyzed tezacaftor/ivacaftor in patients with cystic fibrosis, specifically with the homozygous for Phe508del mutation.[12] The EVOLVE trial is a phase 3, double-blinded, multicenter, randomized, placebo-controlled, parallel-group trial, that was which evaluated therapy with a combination of tezacaftor and ivacaftor in patients that are 12 and older.[12]
510 patients were randomized and 509 patients were given either 100 mg of tezacaftor once daily and 150 mg of ivacaftor twice daily or a placebo for 24 weeks.[12] The combination of drugs was efficacious in patients who had cystic fibrosis with the Phe508del mutation and the adverse effects in both treatment groups were similar.[12]
History
The U.S. Food and Drug Administration (FDA) granted the application for tezacaftor and ivacaftor combination therapy orphan drug designation and priority review, and granted the approval of Symdeko to Vertex Pharmaceuticals Incorporated.[3][13]
References
- ↑ "Symdeko Product information". https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=96881.
- ↑ 2.0 2.1 2.2 "Drug Trials Snapshots: Symdeko". 7 March 2018. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-symdeko.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 "FDA expands approval of treatment for cystic fibrosis to include patients ages 6 and older". U.S. Food and Drug Administration (FDA) (Press release). 21 June 2019. Archived from the original on 21 November 2019. Retrieved 20 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "The combination of tezacaftor and ivacaftor in the treatment of patients with cystic fibrosis: clinical evidence and future prospects in cystic fibrosis therapy". Therapeutic Advances in Respiratory Disease 13: 1753466619844424. 2019. doi:10.1177/1753466619844424. PMID 31027466.
- ↑ "Tezacaftor and ivacaftor for the treatment of cystic fibrosis". Expert Review of Respiratory Medicine 14 (1): 15–30. January 2020. doi:10.1080/17476348.2020.1682998. PMID 31626570. https://www.research.manchester.ac.uk/portal/en/publications/tezacaftor-and-ivacaftor-for-the-treatment-of-cystic-fibrosis(a60081ad-608e-43a3-8e4e-06fe89de284f).html.
- ↑ "The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis". Expert Opinion on Drug Discovery 15 (8): 873–891. August 2020. doi:10.1080/17460441.2020.1750592. PMID 32290721.
- ↑ "Trikafta- elexacaftor, tezacaftor, and ivacaftor kit". 29 January 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f354423a-85c2-41c3-a9db-0f3aee135d8d.
- ↑ "FDA approves new breakthrough therapy for cystic fibrosis". U.S. Food and Drug Administration (FDA) (Press release). October 21, 2019. Archived from the original on November 13, 2019. Retrieved November 13, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Tezacaftor (VX-661) for Cystic Fibrosis". Cystic Fibrosis News Today. Pensacola, FL: BioNews Services, LLC. https://cysticfibrosisnewstoday.com/tezacaftor-vx-661-for-cystic-fibrosis.
- ↑ Ridley, Kaden (2020). "Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy". The Journal of Pediatric Pharmacology and Therapeutics 25 (3): 192–197. doi:10.5863/1551-6776-25.3.192. PMID 32265602.
- ↑ "FDA Approves New Cystic Fibrosis Drug Combo - Symdeko approved for patients with specific CFTR mutations". MedPage Today, LLC. 13 February 2018. https://www.medpagetoday.com/pulmonology/cysticfibrosis/71125.
- ↑ 12.0 12.1 12.2 12.3 Taylor-Cousar, Jennifer (23 November 2017). "Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del". New England Journal of Medicine 377 (21): 2013–2023. doi:10.1056/NEJMoa1709846. PMID 29099344.
- ↑ "Tezacaftor and Ivacaftor Orphan Drug Designations and Approvals". 15 June 2017. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=577517.
External links
- "Tezacaftor". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tezacaftor.
- "Ivacaftor regimen with Tezacaftor". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/1969264-35-4.
 |

