Chemistry:Fibrinopeptide
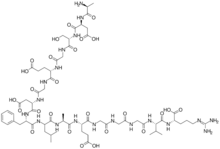
| |
| Names | |
|---|---|
| IUPAC name
(4S)-4-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-aminopropanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-5-[[2-[[(2S)-3-carboxy-1-[[(2S)-1-[[1-[[(2S)-1-[[(2S)-4-carboxy-1-[[2-[[2-[[2-[[(2S)-1-[[(1S)-1-carboxy-4-(diaminomethylideneamino)butyl]amino]-3-methyl-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-2-oxoethyl]amino]-2-oxoethyl]amino]-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-5-oxopentanoic acid
| |
| Other names
Fibrinopeptide A; Fibrinopeptide A (human); FpA; FPA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C63H97N19O26 | |
| Molar mass | 1536.57 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
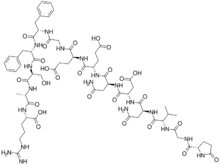
| |
| Names | |
|---|---|
| IUPAC name
(4S)-4-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-3-methyl-2-[[2-[[(2S)-5-oxopyrrolidine-2-carbonyl]amino]acetyl]amino]butanoyl]amino]-4-oxobutanoyl]amino]-3-carboxypropanoyl]amino]-4-oxobutanoyl]amino]-4-carboxybutanoyl]amino]-5-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]amino]-5-oxopentanoic acid
| |
| Other names
Fibrinopeptide B; Fibrinopeptide B (human); FpB; FPB
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C66H93N19O25 | |
| Molar mass | 1552.569 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen (factor I) and are cleaved by the enzyme thrombin (factor IIa) to convert fibrinogen into covalently-linked fibrin (factor IA) monomers.[1][2] The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB.[3][4] Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII (fibrin stabilizing factor), and these fibrin polymers form the backbone of a thrombus (blood clot).[2] Hence, the fibrinopeptides are sensitive markers of fibrinogenesis (fibrin generation), thrombin activity, and coagulation.[5][6][7][8]
FpA is a 16-amino acid peptide.[8] The half-life of FpA is very short at approximately 3 to 5 minutes.[5][8] Hence, FpA levels provide a relatively transient measure of coagulation activation.[8]
Levels of FpA increase with age.[5] FpA levels also gradually increase throughout pregnancy.[9][10] Likewise, FpA levels have been reported to increase with estrogen therapy, including with combined birth control pills and menopausal hormone therapy, although research on FpA levels with these therapies appears to be relatively limited.[11][12][13][7]
References
- ↑ "Fibrinogen and fibrin". Adv Protein Chem. Advances in Protein Chemistry 70: 247–99. 2005. doi:10.1016/S0065-3233(05)70008-5. ISBN 9780120342709. PMID 15837518.
- ↑ 2.0 2.1 "Comparative aspects of blood coagulation". Vet J 168 (3): 238–51. November 2004. doi:10.1016/j.tvjl.2003.09.013. PMID 15501141.
- ↑ "Determinants of fibrin formation, structure, and function". Curr Opin Hematol 19 (5): 349–56. September 2012. doi:10.1097/MOH.0b013e32835673c2. PMID 22759629.
- ↑ O'Riordan, Máiread N; Higgins, John R (June 2003). "Haemostasis in normal and abnormal pregnancy". Best Practice & Research Clinical Obstetrics & Gynaecology 17 (3): 385–396. doi:10.1016/S1521-6934(03)00019-1. ISSN 1521-6934. PMID 12787533.
- ↑ 5.0 5.1 5.2 "Mechanisms, markers and management of coagulation activation". Br Med Bull 50 (4): 851–70. October 1994. doi:10.1093/oxfordjournals.bmb.a072930. PMID 7804735.
- ↑ Vincent Marks; Thomas Cantor; Dusan Mesko; Rudolf Pullmann; Gabriela Nosalova (6 December 2012). Differential Diagnosis by Laboratory Medicine: A Quick Reference for Physicians. Springer Science & Business Media. pp. 443–. ISBN 978-3-642-55600-5. OCLC 1262382180. https://books.google.com/books?id=_VjrCAAAQBAJ&pg=PA443.
- ↑ 7.0 7.1 "Pharmacodynamics of combined estrogen-progestin oral contraceptives: 2. effects on hemostasis". Expert Rev Clin Pharmacol 10 (10): 1129–1144. October 2017. doi:10.1080/17512433.2017.1356718. PMID 28712325.
- ↑ 8.0 8.1 8.2 8.3 "Laboratory Measurement of Thrombin Activity--What Every Clinician Scientist Needs to Know". J Thromb Thrombolysis 2 (2): 85–92. 1995. doi:10.1007/BF01064374. PMID 10608009.
- ↑ "Hemostasis during normal pregnancy and puerperium". Semin Thromb Hemost 29 (2): 125–30. April 2003. doi:10.1055/s-2003-38897. PMID 12709915.
- ↑ Koltsova, Ekaterina; Balandina, Anna; Serebriyskiy, Ilya; Vuimo, Tatiana; Panteleev, Mikhail; Ataullakhanov, Fazoil (21 September 2016). "Classic and Global Hemostasis Testing in Pregnancy and during Pregnancy Complications". Seminars in Thrombosis and Hemostasis 42 (7): 696–716. doi:10.1055/s-0036-1592303. ISSN 0094-6176. PMID 27652600.
- ↑ "Oral Contraceptives and Venous Thromboembolism: Focus on Testing that May Enable Prediction and Assessment of the Risk". Semin Thromb Hemost 46 (8): 872–886. November 2020. doi:10.1055/s-0040-1714140. PMID 33080636.
- ↑ "Hormone therapy and hemostasis among postmenopausal women: a review". Menopause 21 (7): 753–62. July 2014. doi:10.1097/GME.0000000000000296. PMID 24937030. https://www.hal.inserm.fr/inserm-01148743/file/Canonico_Menopause_2014_2.pdf.
- ↑ "The role of estrogen in cardiovascular disease". J Surg Res 115 (2): 325–44. December 2003. doi:10.1016/s0022-4804(03)00215-4. PMID 14697301.
 |
