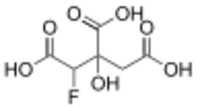
Chemistry:Fluorocitric acid
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3-C-Carboxy-2,4-dideoxy-2-fluoropentaric acid
| |
| Other names
2-Fluorocitric acid; 2-Fluorocitrate; 1-Fluoro-2-hydroxypropane-1,2,3-tricarboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H7FO7 | |
| Molar mass | 210.113 g·mol−1 |
| Appearance | Odorless, white crystals |
| Density | 1.37 |
| Melting point | 35.2°C |
| Boiling point | 165°C |
| Soluble | |
| Hazards | |
| Main hazards |  
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Fluorocitric acid is a fluorinated carboxylic acid derived from citric acid by substitution of one hydrogen by a fluorine atom. The appropriate anion is called fluorocitrate. Fluorocitrate is formed in two steps from fluoroacetate. Fluoroacetate is first converted to fluoroacetyl-CoA by acetyl-CoA synthetase in the mitochondria. Then fluoroacetyl-CoA condenses with oxaloacetate to form fluorocitrate. This step is catalyzed by citrate synthase.[1] Flurocitrate is a metabolite of fluoroacetic acid and is very toxic because it is not processable using aconitase in the citrate cycle (where fluorocitrate takes place of citrate as the substrate). The enzyme is inhibited and the cycle stops working.[2]
See also
- Citric acid
- Fluoroacetic acid
- Citrate cycle
References
- ↑ H., Garrett, Reginald (2013). Biochemistry. Grisham, Charles M. (5th ed.). Belmont, CA: Brooks/Cole, Cengage Learning. ISBN 9781133106296. OCLC 777722371.
- ↑ Horák, J.; Linhart, I.; Klusoň, P. (2004) (in Czech). Úvod do toxikologie a ekologie pro chemiky (1st ed.). Prague: VŠCHT v Praze. ISBN 80-7080-548-X.
External links
- PubChem: Fluorocitrate
- Human Metabolome Database (HMDB): Fluorocitric acid
- The Chemical and Biochemical Properties of Fluorocitric Acid Pdf
 |

