Chemistry:Furanochromone
From HandWiki
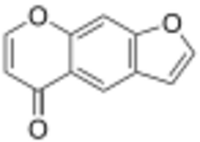
| |
| Names | |
|---|---|
| Preferred IUPAC name
5H-Furo[3,2-g][1]benzopyran-5-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H6O3 | |
| Molar mass | 186.166 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Furanochromone is a chemical compound which is a derivative of chromone (1,4-benzopyrone) and furan.[1]
Some chemical derivatives of furanochromone show strong interaction with DNA.[2] Furanochromones can be produced in callus cultures of Ammi visnaga[3] or in Pimpinella monoica.[4]
References
- ↑ Pereira, David M.; Valentão, Patrícia; Pereira, José A.; Andrade, Paula B. (2009). "Phenolics: From Chemistry to Biology". Molecules 14 (6): 2202–2211. doi:10.3390/molecules14062202.
- ↑ Niccolai, Neri; Bovalini, Lucia; Martelli, Paola (1986). "The mechanisms of interaction between furanochromones and dna". Biophysical Chemistry 24 (3): 217–20. doi:10.1016/0301-4622(86)85027-X. PMID 3768467.
- ↑ Krolicka A.; Staniszewska I.; Malinski E.; Szafranek J.; Łojkowska E. (2003). "Stimulation of furanochromone accumulation in callus cultures of Ammi visnaga L. by addition of elicitors". Pharmazie 58 (8): 590–592. PMID 12967041. http://cat.inist.fr/?aModele=afficheN&cpsidt=15029055.
- ↑ Luthria, D.L.; Banerji, A. (1994). "Biosynthesis of furanochromones in Pimpinella monoica" (PDF). Proceedings of the Indian Academy of Sciences – Chemical Sciences 106 (5): 1149–1156. doi:10.1007/BF02841922. https://www.ias.ac.in/article/fulltext/jcsc/106/05/1149-1156.
 |
