Chemistry:Gallium acetylacetonate
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
(Z)-4-bis[(Z)-1-methyl-3-oxobut-1-enoxy]gallanyloxypent-3-en-2-one
| |
| Other names
Gallium acetylacetonate
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| GaC15H21O6 | |
| Molar mass | 367.05 g/mol |
| Appearance | White solid |
| Density | 1.42 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
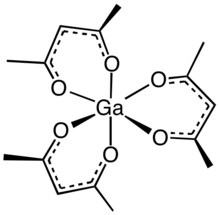
Gallium acetylacetonate, also referred to as Ga(acac)3, is a coordination complex with formula Ga(C5H7O2)3. This gallium complex with three acetylacetonate ligands is used in research. The molecule has D3 symmetry, being isomorphous with other octahedral tris(acetylacetonate)s.[1]
Uses
Gallium oxide thin films can be produced by atomic layer epitaxy (ALE) by combining gallium acetylacetonate with either water or ozone as the precursor.[2] Ga(acac)3 can also be used for low temperature growth of high purity gallium nitride nano-wires and nano-needles.[3][4]
References
- ↑ Dymock, K.; Palenik, G. J. (1974). "Tris(acetylacetonato)gallium(III)". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 30 (5): 1364–1366. doi:10.1107/S0567740874004833.
- ↑ "Growth of gallium oxide thin films from gallium acetylacetonate by atomic layer epitaxy"
- ↑ "Low-Temperature Catalytic Synthesis of Gallium Nitride Nanowires"
- ↑ "Temperature-controlled catalytic growth of one-dimensional Gallium nitride nanostructures using a gallium organometallic precursor"
 |

