Chemistry:Gallium(III) oxide
 β-Ga2O3 crystal
| |
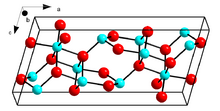 Crystal structure of β-Ga2O3
| |
| Names | |
|---|---|
| Other names
gallium trioxide, gallium sesquioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| Ga2O3 | |
| Molar mass | 187.444 g/mol |
| Appearance | white crystalline powder |
| Melting point | 1,725 °C (3,137 °F; 1,998 K)[1] |
| insoluble | |
| Solubility | soluble in most acids |
| Structure[2][3] | |
| Monoclinic, mS20, space group = C2/m, No. 12 | |
a = 1.2232 nm, b = 0.3041 nm, c = 0.5801 nm α = 90°, β = 103.73°, γ = 90° β-phase
| |
Formula units (Z)
|
4 |
| Thermochemistry[4] | |
Heat capacity (C)
|
92.1 J/(mol·K) |
Std molar
entropy (S |
85.0 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−1089.1 kJ/mol |
Gibbs free energy (ΔfG˚)
|
−998.3 kJ/mol |
Enthalpy of fusion (ΔfH⦵fus)
|
100 kJ/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gallium(III) oxide is an inorganic compound and ultra-wide-bandgap semiconductor with the formula Ga2O3. It is actively studied for applications in power electronics, phosphors, and gas sensing.[5][6][7] The compound has several polymorphs, of which the monoclinic β-phase is the most stable. The β-phase’s bandgap of 4.7–4.9 eV and large-area, native substrates make it a promising competitor to GaN and SiC-based power electronics applications and solar-blind UV photodetectors.[7][8] The orthorhombic ĸ-Ga2O3 is the second most stable polymorph. The ĸ-phase has shown instability of subsurface doping density under thermal exposure.[9] Ga2O3 exhibits reduced thermal conductivity and electron mobility by an order of magnitude compared to GaN and SiC, but is predicted to be significantly more cost-effective due to being the only wide-bandgap material capable of being grown from melt.[7][10][11] β-Ga2O3 is thought to be radiation-hard, which makes it promising for military and space applications.[12][13]
Preparation
Gallium trioxide is precipitated in hydrated form upon neutralization of acidic or basic solution of gallium salt. Also, it is formed on heating gallium in air or by thermally decomposing gallium nitrate at 200–250 °C.
Crystalline Ga2O3 can occur in five polymorphs, α, β, γ, δ, and ε. Of these polymorphs β-Ga2O3 is the most thermodynamically stable phase at standard temperature and pressure[14] while α-Ga2O3 is the most stable polymorph under high pressures.[15]
- β-Ga2O3 epitaxial thin films can be deposited heteroepitaxially on substrates such as sapphire, GaN, SiC, and Si, as well as homoepitaxially. For example, ALD on sapphire substrates at temperatures between 190 °C and 550 °C have been demonstrated.[16] High-quality β-Ga2O3 films have also been grown using techniques such as MBE, HVPE, and MOVPE.[17] HVPE is preferred for vertical power semiconductor devices due to its fast growth rate.[18] β-Ga2O3 epitaxial films grown by MOVPE exhibit higher electron mobilities and lower background carrier concentrations than those grown by other thin-film growth techniques.[19][20]
Bulk substrates of β-Ga2O3 can be produced, which is one of the major advantages of this material system. Bulk substrates can be produced in multiple orientations and by multiple techniques.[21][22]

- α-Ga2O3 can be obtained by heating β-Ga2O3 at 65 kbar and 1100 °C. It has a corundum structure. The hydrated form can be prepared by decomposing precipitated and "aged" gallium hydroxide at 500 °C. Epitaxial thin films of α-Ga2O3 deposited on c-plane (0001), m-plane (1010), or a-plane (1120) sapphire substrates have been demonstrated.
- γ-Ga2O3 is prepared by rapidly heating the hydroxide gel at 400–500 °C. A more crystalline form of this polymorph can be prepared directly from gallium metal by a solvothermal synthesis.[23]
- δ-Ga2O3 is obtained by heating Ga(NO3)3 at 250 °C.[24]
- ε-Ga2O3 is prepared by heating δ-Ga2O3 at 550 °C.[14] Thin films of ε-Ga2O3 are deposited by means of metalorganic vapour-phase epitaxy using trimethylgallium and water on sapphire substrates at temperatures between 550 and 650 °C[25]
Reactions
Gallium(III) trioxide is amphoteric.[26] It reacts with alkali metal oxides at high temperature to form, e.g., NaGaO2, and with Mg, Zn, Co, Ni, Cu oxides to form spinels, e.g., MgGa2O4.[27] It dissolves in strong alkali to form a solution of the gallate ion, Ga(OH)−4.
With HCl, it forms gallium trichloride GaCl3.[28]
- Ga2O3 + 6 HCl → 2 GaCl3 + 3 H2O
It can be reduced to gallium suboxide (gallium(I) oxide) Ga2O by H2.[29] or by reaction with gallium metal:[30]
- Ga2O3 + 2 H2 → Ga2O + 2 H2O
- Ga2O3 + 4 Ga → 3 Ga2O
Structure
β-Ga2O3, with a melting point of 1900 °C, is the most stable crystalline modification. The oxide ions are in a distorted cubic closest packing arrangement, and the gallium (III) ions occupy distorted tetrahedral and octahedral sites, with Ga–O bond distances of 1.83 and 2.00 Å respectively.[31]
α-Ga2O3 has the same structure (corundum) as α-Al2O3, wherein Ga ions are 6-coordinate.[32][33]
γ-Ga2O3 has a defect spinel structure similar to that of γ-Al2O3.[34]
ε-Ga2O3 films deposited by metalorganic vapour-phase epitaxy show a columnar structure with orthorhombic crystal symmetry. Macroscopically, this structure is seen by X-ray crystallography as hexagonal close packed.[35]
κ-Ga2O3 has an orthorhombic structure and forms with 120° twin domains, resulting in hexagonal symmetry which is often identified as ε-Ga2O3.[36]
| Phase of Ga2O3 | Figure | Crystal structure name |
|---|---|---|
| α |  |
Rhombohedral
(Corundum) |
| β | 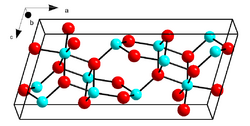 |
Monoclinic |
| γ |  |
Cubic defect spinel |
| δ |  |
Body-centered cubic bixbyite |
| ε |  |
Hexagonal |
| κ (subgroup of ε phase)[41] |  |
Orthorhombic |
Applications
Gallium(III) oxide has been studied for usage as passive components in lasers,[43] phosphors,[5] and luminescent materials[44] as well as active components for gas sensors,[6] power diodes,[45] and power transistors.[46][47] Since the first publication in January 2012 by the National Institute of Information and Communications Technology, in collaboration with Tamura Co., Ltd. and Koha Co., Ltd. of the world's first single-crystal gallium oxide (Ga2O3) field-effect transistors, the predominant interest in gallium oxide is in the β-polymorph for power electronics.[48][7]
Monoclinic β-Ga2O3 has shown increasing performance since 2012 approaching state of the art GaN and SiC power devices.[7] β-Ga2O3 Schottky diodes have exceeded breakdown voltages of 2400 V.[45] β-Ga2O3/NiOx p–n diodes have exhibited breakdown voltages over 1200 V.[49] β-Ga2O3 MOSFETs have individually achieved figures of merits of fT of 27 GHz,[46] fMAX of 48 GHz,[47] and 5.4 MV/cm average breakdown field.[47] This field exceeds that which is possible in SiC or GaN.
ε-Ga2O3 thin films deposited on sapphire show potential applications as solar-blind UV photodetector.[8]
References
- ↑ Patnaik, Pradyot (2002) Handbook of Inorganic Chemicals. McGraw-Hill. ISBN:0-07-049439-8.
- ↑ Dohy, D.; Gavarri, J. R. (1983). "Oxyde β-Ga2O3: Champ de force, dilatation thermique, et rigidité anisotropes". Journal of Solid State Chemistry 49 (1): 107–117. doi:10.1016/0022-4596(83)90222-0. Bibcode: 1983JSSCh..49..107D.
- ↑ Geller, S. (1 September 1960). "Crystal Structure of β‐Ga2O3". The Journal of Chemical Physics 33 (3): 676–684. doi:10.1063/1.1731237. Bibcode: 1960JChPh..33..676G.
- ↑ Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. pp. 5.20, 6.157. ISBN 1439855110.
- ↑ 5.0 5.1 Lin, Jianhua; Zhou, Liuyan; Shen, Yuyu; Fu, Jie; Chen, Yanling; Lei, Lei; Ye, Renguang; Shen, Yang et al. (2022-12-10). "[Zn2+-Ge4+] co-substitutes [Ga3+-Ga3+] to coordinately broaden the near-infrared emission of Cr3+ in Ga2O3 phosphors". Physical Chemistry Chemical Physics 25 (3): 2090–2097. doi:10.1039/D2CP04737C. PMID 36562283.
- ↑ 6.0 6.1 Liu, Zhifu; Yamazaki, Toshinari; Shen, Yanbai; Kikuta, Toshio; Nakatani, Noriyuki; Li, Yongxiang (2008-02-22). "O2 and CO sensing of Ga2O3 multiple nanowire gas sensors". Sensors and Actuators B: Chemical 129 (2): 666–670. doi:10.1016/j.snb.2007.09.055.
- ↑ 7.0 7.1 7.2 7.3 7.4 Green, Andrew J.; Speck, James; Xing, Grace; Moens, Peter; Allerstam, Fredrik; Gumaelius, Krister; Neyer, Thomas; Arias-Purdue, Andrea et al. (2022-02-01). "β-Gallium oxide power electronics". APL Materials 10 (2): 029201. doi:10.1063/5.0060327. Bibcode: 2022APLM...10b9201G.
- ↑ 8.0 8.1 Pavesi, M. (2018). "ε-Ga2O3 epilayers as a material for solar-blind UV photodetectors". Materials Chemistry and Physics 205: 502–507. doi:10.1016/j.matchemphys.2017.11.023.
- ↑ Rajabi Kalvani, Payam; Parisini, Antonella; Sozzi, Giovanna; Borelli, Carmine; Mazzolini, Piero; Bierwagen, Oliver; Vantaggio, Salvatore; Egbo, Kingsley et al. (2023-10-04). "Interfacial Properties of the SnO/κ-Ga 2 O 3 p-n Heterojunction: A Case of Subsurface Doping Density Reduction via Thermal Treatment in κ-Ga 2 O 3" (in en). ACS Applied Materials & Interfaces 15 (39): 45997–46009. doi:10.1021/acsami.3c08841. ISSN 1944-8244. PMID 37733937.
- ↑ Reese, Samantha B.; Remo, Timothy; Green, Johney; Zakutayev, Andriy (2019-04-17). "How Much Will Gallium Oxide Power Electronics Cost?". Joule 3 (4): 903–907. doi:10.1016/j.joule.2019.01.011.
- ↑ Heinselman, Karen N.; Haven, Drew; Zakutayev, Andriy; Reese, Samantha B. (2022-08-03). "Projected Cost of Gallium Oxide Wafers from Edge-Defined Film-Fed Crystal Growth". Crystal Growth & Design 22 (8): 4854–4863. doi:10.1021/acs.cgd.2c00340.
- ↑ Bauman, D. A.; Borodkin, A. I.; Petrenko, A. A.; Panov, D. I.; Kremleva, A. V.; Spiridonov, V. A.; Zakgeim, D. A.; Silnikov, M. V. et al. (2021-03-01). "On improving the radiation resistance of gallium oxide for space applications". Acta Astronautica 180: 125–129. doi:10.1016/j.actaastro.2020.12.010. Bibcode: 2021AcAau.180..125B.
- ↑ Pearton, Stephen J.; Ren, Fan; Law, Mark; Mastro, Michael (2021). Fundamental Studies and Modeling of Radiation Effects in beta-Gallium Oxide. Defense Threat Reduction Agency, U.S.. https://apps.dtic.mil/sti/citations/trecms/AD1123735.
- ↑ 14.0 14.1 Bailar, J; Emeléus, H; Nyholm, R; Trotman-Dickenson, A. F. (1973). Comprehensive Inorganic Chemistry. Vol. 1, p. 1091.
- ↑ Yan-Mei et alChinese Phys. Lett. 25 1603, Ma (2008). "High-Pressure and High-Temperature Behaviour of Gallium Oxide". Chinese Physics Letters 25 (5): 1603–1605. doi:10.1088/0256-307X/25/5/022.
- ↑ Rafie Borujeny, E.; Sendetskyi, O.; Fleischauer, M. D.; Cadien, K. C. (2020). "Low Thermal Budget Heteroepitaxial Gallium Oxide Thin Films Enabled by Atomic Layer Deposition". ACS Applied Materials & Interfaces 12 (39): 44225–44237. doi:10.1021/acsami.0c08477. PMID 32865966.
- ↑ Green, Andrew J.; Speck, James; Xing, Grace; Moens, Peter; Allerstam, Fredrik; Gumaelius, Krister; Neyer, Thomas; Arias-Purdue, Andrea et al. (2022-02-01). "β-Gallium oxide power electronics". APL Materials 10 (2): 029201. doi:10.1063/5.0060327. Bibcode: 2022APLM...10b9201G.
- ↑ Yang, Duyoung; Kim, Byungsoo; Eom, Tae Hoon; Park, Yongjo; Jang, Ho Won (2022-03-01). "Epitaxial Growth of Alpha Gallium Oxide Thin Films on Sapphire Substrates for Electronic and Optoelectronic Devices: Progress and Perspective". Electronic Materials Letters 18 (2): 113–128. doi:10.1007/s13391-021-00333-5. Bibcode: 2022EML....18..113Y.
- ↑ Tsao, J. Y.; Chowdhury, S.; Hollis, M. A.; Jena, D.; Johnson, N. M.; Jones, K. A.; Kaplar, R. J.; Rajan, S. et al. (2018). "Ultrawide‐Bandgap Semiconductors: Research Opportunities and Challenges". Advanced Electronic Materials 4 (1): 1600501. doi:10.1002/aelm.201600501.
- ↑ Zhang, Yuewei; Alema, Fikadu; Mauze, Akhil; Koksaldi, Onur S.; Miller, Ross; Osinsky, Andrei; Speck, James S. (2019-02-01). "MOCVD grown epitaxial β-Ga2O3 thin film with an electron mobility of 176 cm2/V s at room temperature". APL Materials 7 (2): 022506. doi:10.1063/1.5058059. Bibcode: 2019APLM....7b2506Z.
- ↑ Galazka, Zbigniew (2022-01-21). "Growth of bulk β-Ga2O3 single crystals by the Czochralski method". Journal of Applied Physics 131 (3): 031103. doi:10.1063/5.0076962. Bibcode: 2022JAP...131c1103G.
- ↑ "Substrates". https://www.novelcrystal.co.jp/eng/substrates/.
- ↑ Playford, Helen Y.; Hannon, Alex C.; Barney, Emma R.; Walton, Richard I. (2013). "Structures of Uncharacterised Polymorphs of Gallium Trioxide from Total Neutron Diffraction". Chemistry: A European Journal 19 (8): 2803–13. doi:10.1002/chem.201203359. PMID 23307528.
- ↑ Li, Liandi; Wei, Wei; Behrens, Malte (July 2012). "Synthesis and characterization of α-, β-, and γ-Ga2O3 prepared from aqueous solutions by controlled precipitation". Solid State Sciences 14 (7): 971–981. doi:10.1016/j.solidstatesciences.2012.04.037. Bibcode: 2012SSSci..14..971L.
- ↑ Boschi, F.; Bosi, M.; Berzina, T.; Buffagni, E.; Ferrari, C.; Fornari, R. (2015). "Hetero-epitaxy of ε-Ga2O3 layers by MOCVD and ALD". Journal of Crystal Growth 44: 25–30. doi:10.1016/j.jcrysgro.2016.03.013.
- ↑ Ebbing, Darrell D.; Gammon, Steven D. (2010) General Chemistry, 9th ed., Thomson Brooks/Cole. ISBN:0538497521
- ↑ Downs, Anthony John (ed.) (1993) The Chemistry of Aluminium, Gallium, Indium and Thallium. Springer. ISBN:075140103X
- ↑ Zuckerman, J J and Hagen, A P eds. (2009) Inorganic Reactions and Methods, the Formation of Bonds to Halogens (Part 2), Wiley-VCH Verlag GmbH, ISBN:9780470145395
- ↑ Koch, H. F.; Girard, L. A.; Roundhill, D. M. (1999). "Determination of Gallium in a Cerium Surrogate and in Drops from a Copper Collector by ICP as Model Studies for the Removal of Gallium from Plutonium". Atomic Spectroscopy 20 (1): 30.
- ↑ Greenwood, N.N.; Emeleus, H. J. and Sharpe, A. G. (1963) "The chemistry of Gallium" in Advances in Inorganic Chemistry and Radiochemistry, Vol. 5, Elsevier, Academic Press
- ↑ King, R. B. (1994) Encyclopedia of Inorganic Chemistry. Vol. 3. p. 1256. ISBN:978-0-470-86078-6.
- ↑ Eckert, L. J.; Bradt, R. C. (1973). "Thermal Expansion of Alpha Ga2O3". Journal of the American Ceramic Society 56 (4): 229. doi:10.1111/j.1151-2916.1973.tb12471.x.
- ↑ 33.0 33.1 Marezio, M.; Remeika, J. P. (1 March 1967). "Bond Lengths in the α‐Ga2O3 Structure and the High‐Pressure Phase of Ga2−xFexO3". The Journal of Chemical Physics 46 (5): 1862–1865. doi:10.1063/1.1840945.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 247. ISBN 978-0-08-037941-8.
- ↑ Cora, I (2017). "The real structure of ε-Ga2O3 and its relation to κ-phase". CrystEngComm 19 (11): 1509–1516. doi:10.1039/C7CE00123A.
- ↑ Fornari, R.; Pavesi, M.; Montedoro, V.; Klimm, D.; Mezzadri, F.; Cora, I.; Pécz, B.; Boschi, F. et al. (2017-11-01). "Thermal stability of ε-Ga2O3 polymorph". Acta Materialia 140: 411–416. doi:10.1016/j.actamat.2017.08.062. Bibcode: 2017AcMat.140..411F.
- ↑ Playford, Helen. Y.; Hannon, Alex C.; Tucker, Matthew G.; Dawson, Daniel M.; Ashbrook, Sharon E.; Kastiban, Reza J.; Sloan, Jeremy; Walton, Richard I. (2014-07-24). "Characterization of Structural Disorder in γ-Ga2O3". The Journal of Physical Chemistry C 118 (29): 16188–16198. doi:10.1021/jp5033806.
- ↑ Roy, Rustum; Hill, V. G.; Osborn, E. F. (1952). "Polymorphism of Ga2O3 and the System Ga2O3—H2O". Journal of the American Chemical Society 74 (3): 719–722. doi:10.1021/ja01123a039.
- ↑ Prewitt, Charles T.; Shannon, Robert D.; Rogers, Donald Burl; Sleight, Arthur W. (1969). "C rare earth oxide-corundum transition and crystal chemistry of oxides having the corundum structure". Inorganic Chemistry 8 (9): 1985–1993. doi:10.1021/ic50079a033.
- ↑ Playford, Helen Y.; Hannon, Alex C.; Barney, Emma R.; Walton, Richard I. (2013-02-18). "Structures of Uncharacterised Polymorphs of Gallium Oxide from Total Neutron Diffraction". Chemistry: A European Journal 19 (8): 2803–2813. doi:10.1002/chem.201203359. PMID 23307528.
- ↑ Spencer, Joseph A.; Mock, Alyssa L.; Jacobs, Alan G.; Schubert, Mathias; Zhang, Yuhao; Tadjer, Marko J. (2022-03-01). "A review of band structure and material properties of transparent conducting and semiconducting oxides: Ga2O3, Al2O3, In2O3, ZnO, SnO2, CdO, NiO, CuO, and Sc2O3". Applied Physics Reviews 9 (1): 011315. doi:10.1063/5.0078037. Bibcode: 2022ApPRv...9a1315S.
- ↑ Cora, Ildikó; Mezzadri, Francesco; Boschi, Francesco; Bosi, Matteo; Čaplovičová, Maria; Calestani, Gianluca; Dódony, István; Pécz, Béla et al. (2017-03-13). "The real structure of ε-Ga2O3 and its relation to κ-phase". CrystEngComm 19 (11): 1509–1516. doi:10.1039/C7CE00123A.
- ↑ Deng, Huiyang; Leedle, Kenneth J.; Miao, Yu; Black, Dylan S.; Urbanek, Karel E.; McNeur, Joshua; Kozák, Martin; Ceballos, Andrew et al. (April 2020). "Ga2O3‐Based Optical Applications: Gallium Oxide for High‐Power Optical Applications (Advanced Optical Materials 7/2020)". Advanced Optical Materials 8 (7): 2070026. doi:10.1002/adom.202070026.
- ↑ Nogales, E; Méndez, B; Piqueras, J; García, J A (2009-03-18). "Europium doped gallium oxide nanostructures for room temperature luminescent photonic devices". Nanotechnology 20 (11): 115201. doi:10.1088/0957-4484/20/11/115201. PMID 19420434. Bibcode: 2009Nanot..20k5201N. https://eprints.ucm.es/id/eprint/24274/1/MendezBianchi15.pdf.
- ↑ 45.0 45.1 Li, Wenshen; Nomoto, Kazuki; Hu, Zongyang; Jena, Debdeep; Xing, Huili Grace (January 2020). "Field-Plated Ga2O3 Trench Schottky Barrier Diodes With a BV2/Ron,sp of up to 0.95 GW/cm2". IEEE Electron Device Letters 41 (1): 107–110. doi:10.1109/LED.2019.2953559.
- ↑ 46.0 46.1 Xia, Zhanbo; Xue, Hao; Joishi, Chandan; Mcglone, Joe; Kalarickal, Nidhin Kurian; Sohel, Shahadat H.; Brenner, Mark; Arehart, Aaron et al. (July 2019). "β-Ga2O3 Delta-Doped Field-Effect Transistors With Current Gain Cutoff Frequency of 27 GHz". IEEE Electron Device Letters 40 (7): 1052–1055. doi:10.1109/LED.2019.2920366. Bibcode: 2019IEDL...40.1052X.
- ↑ 47.0 47.1 47.2 Saha, Chinmoy Nath; Vaidya, Abhishek; Bhuiyan, A. F. M. Anhar Uddin; Meng, Lingyu; Zhao, Hongping; Singisetti, Uttam (2022-11-02). "Beta-Ga2O3 MOSFETs with near 50 GHz fMAX and 5.4 MV/cm average breakdown field". arXiv:2211.01088 [cond-mat.mtrl-sci].
- ↑ Higashiwaki, Masataka; Sasaki, Kohei; Kuramata, Akito; Masui, Takekazu; Yamakoshi, Shigenobu (2012-01-02). "Gallium oxide (Ga2O3) metal-semiconductor field-effect transistors on single-crystal β-Ga2O3 (010) substrates". Applied Physics Letters 100 (1): 013504. doi:10.1063/1.3674287. Bibcode: 2012ApPhL.100a3504H.
- ↑ Wang, Chenlu; Gong, Hehe; Lei, Weina; Cai, Yuncong; Hu, Zhuangzhuang; Xu, Shengrui; Liu, Zhihong; Feng, Qian et al. (April 2021). "Demonstration of the p-NiOx/n-Ga2O3 Heterojunction Gate FETs and Diodes With BV2/Ron,sp Figures of Merit of 0.39 GW/cm2 and 1.38 GW/cm2". IEEE Electron Device Letters 42 (4): 485–488. doi:10.1109/LED.2021.3062851. Bibcode: 2021IEDL...42..485W.
 |

