Chemistry:Gallium(III) iodide
From HandWiki
| |

| |
| Names | |
|---|---|
| Other names
gallium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| GaI3 | |
| Molar mass | 450.436 g/mol |
| Appearance | light yellow powder |
| Density | 4.5 g/cm3[1] |
| Melting point | 212 °C (414 °F; 485 K)[1] |
| Boiling point | 340 °C (644 °F; 613 K)[1] |
| decomposes | |
| −149.0·10−6 cm3/mol | |
| Thermochemistry[2] | |
Heat capacity (C)
|
100 J/(mol·K) |
Std molar
entropy (S |
205.0 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−238.9 kJ/mol |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H314, H317, H334, H335, H361 | |
| P280, P305+351+338, P310 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
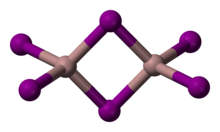
Gallium(III) iodide is the inorganic compound with the formula GaI3. A yellow hygroscopic solid, it is the most common iodide of gallium.[3] In the chemical vapor transport method of growing crystals of gallium arsenide uses iodine as the transport agent. In the solid state, it exists as the dimer Ga2I6.[4] When vaporized, its forms GaI3 molecules of D3h symmetry where the Ga–I distance is 2.458 Angstroms.[5]
Gallium triiodide can be reduced with gallium metal to give a green-colored gallium(I) iodide. The nature of this species is unclear, but it is useful for the preparation of gallium(I) and gallium(II) compounds.[6][7]
See also
References
- ↑ 1.0 1.1 1.2 Haynes, p. 4.63
- ↑ Haynes, p. 5.20
- ↑ Donges, E. (1963). "Gallium(III) Iodide". in Brauer, G.. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 846.
- ↑ Brünig, C.; Locmelis, S.; Milke, E.; Binnewies, M. (2006). "Chemischer Transport fester Lösungen. 27. Mischphasenbildung und chemischer Transport im System Zn Se/Ga As". Zeitschrift für Anorganische und Allgemeine Chemie 632 (6): 1067–1072. doi:10.1002/zaac.200600008.
- ↑ Haynes, p. 9.23
- ↑ Baker, Robert J.; Jones, Cameron (2005). ""GaI": A versatile reagent for the synthetic chemist". Dalton Trans (8): 1341–1348. doi:10.1039/b501310k. PMID 15824768.
- ↑ Green, Shaun P.; Jones, Cameron; Stasch, Andreas; Rose, Richard P. (2007). "'GaI': A new reagent for chemo- and diastereoselective C–C bond forming reactions". New J. Chem. 31: 127–134. doi:10.1039/b613669a.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |

