Chemistry:Hagemann's ester
| |
| Names | |
|---|---|
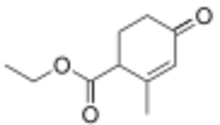
| Preferred IUPAC name
Ethyl 2-methyl-4-oxocyclohex-2-ene-1-carboxylate | |
| Other names
Ethyl 2-methyl-4-oxocyclohex-2-enecarboxylate
4-Carbethoxy-3-methyl-2-cyclohexen-1-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[1] | |
| C10H14O3 | |
| Molar mass | 182.219 g·mol−1 |
| Density | 1.078 g/mL |
| Boiling point | 268 to 272 °C (514 to 522 °F; 541 to 545 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hagemann's ester, ethyl 2-methyl-4-oxo-2-cyclohexenecarboxylate, is an organic compound that was first prepared and described in 1893 by German chemist Carl Hagemann. The compound is used in organic chemistry as a reagent in the synthesis of many natural products including sterols, trisporic acids, and terpenoids.
Preparation
Hagemann's approach
Methylene iodide and two equivalents of ethyl acetoacetate react in the presence of sodium methoxide to form the diethyl ester of 2,4-diacetyl pentane. This precursor is treated with base to induce cyclization. Finally, heat is applied to generate Hagemann's ester.[2][3]
Knoevenagel's approach
Soon after Hagemann, Emil Knoevenagel described a modified procedure to produce the same intermediate diethyl ester of 2,4-diacetyl pentane using formaldehyde and two equivalents of ethyl acetoacetate which undergo condensation in the presence of a catalytic amount of piperidine.[3]
Newman and Lloyd approach
2-Methoxy-1,3-butadiene and ethyl-2-butynoate undergo a Diels-Alder reaction to generate a precursor which is hydrolyzed to obtain Hagemann's ester. By varying the substituents on the butynoate starting material, this approach allows for different C2 alkylated Hagemann's ester derivatives to be synthesized.[3]
Mannich and Forneau approach
Original
Methyl vinyl ketone, ethyl acetoacetate, and diethyl-methyl-(3-oxo-butyl)-ammonium iodide react to form a cyclic aldol product. Sodium methoxide is added to generate Hagemann's ester.
Variations
Methyl vinyl ketone and ethyl acetoacetate undergo aldol cyclization in the presence of catalytic pyrrolidinum acetate or Triton B or sodium ethoxide to produce Hagemann's ester.[3] This variant is a type of Robinson annulation.[4]
Uses
Hagemann's ester has been used as a key building block in many syntheses.[3] For example, a key intermediate for the fungal hormone trisporic acid was made by its alkylation[5] and it has been used to make sterols.[6] Other authors have used it in inverse-electron-demand Diels–Alder reactions leading to sesquiterpene dimers[7] or in reactions forming simple derivatives.[8][9][10]
References
- ↑ 2-methyl-4-oxo-2-cyclohexenecarboxylate at Sigma-Aldrich
- ↑ Hagemann, C. Th. L. (1893). "Ueber die Einwirkung von Methylenjodid auf Natracetessigäther" (in de). Berichte der Deutschen Chemischen Gesellschaft 26: 876–890. doi:10.1002/cber.189302601181. https://zenodo.org/record/2026697.
- ↑ 3.0 3.1 3.2 3.3 3.4 Pollini, Gian Piero; Benetti, Simonetta; De Risi, Carmela; Zanirato, Vinicio (2010). "Hagemann's ester: a timeless building block for natural product synthesis". Tetrahedron 66 (15): 2775–2802. doi:10.1016/j.tet.2010.01.078.
- ↑ Rapson, William Sage; Robinson, Robert (1935). "307. Experiments on the synthesis of substances related to the sterols. Part II. A new general method for the synthesis of substituted cyclohexenones". Journal of the Chemical Society (Resumed): 1285. doi:10.1039/JR9350001285.
- ↑ White, James D.; Sung, Wing Lam (1974). "Alkylation of Hagemann's ester. Preparation of an intermediate for trisporic acid synthesis". The Journal of Organic Chemistry 39 (16): 2323–2328. doi:10.1021/jo00930a001.
- ↑ Hogg, John A. (1948). "Synthetic Sterols. I. Model Experiments Employing Hagemann's Ester". Journal of the American Chemical Society 70 (1): 161–164. doi:10.1021/ja01181a047. PMID 18918810.
- ↑ Liu, Bo; Yang, Li; Yue, Guizhou; Yuan, Changchun; Du, Biao; Deng, Heping (2014). "Synthetic Studies toward Lindenane-Type Sesquiterpenoid Dimers". Synlett 25 (17): 2471–2474. doi:10.1055/s-0034-1379001.
- ↑ McAndrew, Bruce A. (1979). "Ethyl 2- methyl-4-oxocyclohex-2-enecarboxylate (Hagemann's ester) as a precursor to alkyl-substituted 3-methylcyclohexenones". Journal of the Chemical Society, Perkin Transactions 1: 1837. doi:10.1039/P19790001837.
- ↑ Nasipuri, D.; Mitra, K.; Venkataraman, S. (1972). "Cyclohexenone derivatives. Part VI. C-3 and C-1 alkylation of Hagemann's ester (Ethyl 2-methyl-4-oxocyclohex-2-enecarboxylate) with alkyl halides and Michael acceptors". Journal of the Chemical Society, Perkin Transactions 1: 1836. doi:10.1039/P19720001836.
- ↑ Begbie, A. L.; Golding, B. T. (1972). "A new synthesis of ethyl 2-methyl-4-oxocyclohex-2-enecarboxylate (Hagemann's ester) and its methyl and t-butyl analogues". Journal of the Chemical Society, Perkin Transactions 1: 602. doi:10.1039/P19720000602.
 |

