Chemistry:Hexachloropropene
From HandWiki
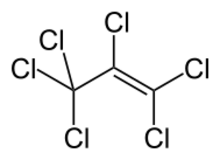
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,2,3,3,3-Hexachloroprop-1-ene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3382 3082 |
| |
| |
| Properties | |
| C3Cl6 | |
| Molar mass | 248.75 g/mol |
| Appearance | colourless liquid[1] |
| Density | 1.765 g/cm3 (at 25 °C) |
| Melting point | −73[1] °C (−99 °F; 200 K) |
| Boiling point | 209–210[1] °C (408–410 °F; 482–483 K) |
| 0.25 g/L[1] | |
| Solubility | soluble in carbon tetrachloride, ethanol and diethyl ether[2] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H315, H319, H330, H332, H335 | |
| P260, P261, P264, P271, P280, P284, P302+352, P304+312, P304+340, P305+351+338, P310, P312, P320, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexachloropropene is a toxic compound of chlorine and carbon. Its linear formula is CCl3CCl=CCl2.[3]
Hexachloropropene can be produced by the elimination reaction of 1,1,1,2,2,3,3-heptachloropropane by potassium hydroxide in methanol solution.[4]
Hexachloropropene can be used to produce other compounds such as uranium tetrachloride, anhydrous niobium pentachloride and tungsten hexachloride.[5]
References
- ↑ 1.0 1.1 1.2 1.3 Hexachlorpropen
- ↑ Gangolli, S.; Royal Society of Chemistry (1999). The dictionary of substances and their effects.. Cambridge, UK. p. 607. ISBN 0-85404-803-0. OCLC 41660040.
- ↑ "Hexachloropropene96%". Sigma Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/h6401?lang=en®ion=. Retrieved Nov 27, 2013.
- ↑ Friedrich Boberg (1964-11-16). "Über 1.2-Dithia-cyclopentene, V. 4.5-Dichlor-1.2-dithia-cyclopentenon-(3)" (in de). Justus Liebigs Annalen der Chemie 679 (1): 109–118. doi:10.1002/jlac.19646790115. https://onlinelibrary.wiley.com/doi/10.1002/jlac.19646790115. Retrieved 2022-03-08.
- ↑ W. W. Porterfield and S. Y. Tyree, Jr. (1967), S. Young Tyree, Jr., ed. (in en), Anhydrous metal chlorides, McGraw-Hill Book Company, Inc., pp. 133–136

