Chemistry:Tungsten hexachloride
 α-Tungsten hexachloride
| |||
 β-Tungsten hexachloride
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Tungsten hexachloride
Tungsten(VI) chloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| WCl 6 | |||
| Molar mass | 396.54 g·mol−1 | ||
| Appearance | dark blue crystals, moisture sensitive | ||
| Density | 3.52 g/cm3 | ||
| Melting point | 275 °C (527 °F; 548 K) | ||
| Boiling point | 346.7 °C (656.1 °F; 619.8 K) | ||
| Hydrolyzes | |||
| Solubility in chlorocarbons | soluble | ||
| −71.0·10−6 cm3/mol | |||
| Structure | |||
| α:rhombohedral, β: hexagonal | |||
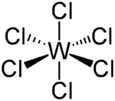
| Octahedral | |||
| 0 D | |||
| Hazards | |||
| Main hazards | oxidizer; hydrolysis releases HCl | ||
| Related compounds | |||
Other anions
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula WCl
6. This dark violet blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds.[1] Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.
As a d0 atom, tungsten hexachloride is diamagnetic.
Preparation and structure
Tungsten hexachloride can be prepared by chlorinating tungsten metal in a sealed tube at 600 °C:[2]
- W + 3 Cl
2 → WCl
6
Tungsten hexachloride exists in both blue and red polymorphs, referred to respectively as α and β. The wine-red β can be obtained by rapid cooling whereas the blue α form is more stable at room temperature. Although these polymorphs are distinctly colored, their molecular structures are very similar. Both polymorphs feature WCl
6 molecules that have octahedral, in which all six W–Cl bonds are equivalent, and their length is equal to 224–226 pm. The densities are very similar: 3.68 g/cm3 for α and 3.62 g/cm3 for β. The low temperature form is slightly more dense, as expected.[3]
Reactions
Tungsten hexachloride is readily hydrolyzed, even by moist air, giving the orange oxychlorides WOCl
4 and WO
2Cl
2, and subsequently, tungsten trioxide. WCl
6 is soluble in carbon disulfide, carbon tetrachloride, and phosphorus oxychloride.[2]
Methylation with trimethylaluminium affords hexamethyl tungsten:
- WCl
6 + 3 Al
2(CH
3)
6 → W(CH
3)
6 + 3 Al
2(CH
3)
4Cl
2
Treatment with butyl lithium affords a reagent that is useful for deoxygenation of epoxides.[4]
The chloride ligands in WCl
6 can be replaced by many anionic ligands including: bromide, thiocyanate, alkoxide, alkyl and aryl).
Reduction of WCl
6 can be effected with a mixture of tetrachloroethylene and tetraphenylarsonium chloride:[5]
- 2 WCl
6 + Cl
2C=CCl
2 + 2 (C
6H
5)
4AsCl → 2 (C
6H
5)
4As[WCl
6] + Cl
3C–CCl
3
The W(V) hexachloride is a derivative of tungsten(V) chloride.
It reacts with arsenic or hydrogen arsenide to form tungsten arsenide.[6][7]
Safety considerations
WCl
6 is an aggressively corrosive oxidant, and hydrolyzes to release hydrogen chloride.
References
- ↑ J. W. Herndon; M. E. Jung (2007). "Encyclopedia of Reagents for Organic Synthesis". Encyclopedia of Reagents for Organic Synthesis. Wiley. doi:10.1002/9780470842898.rt430.pub2. ISBN 978-0471936237..
- ↑ Jump up to: 2.0 2.1 M. H. Lietzke; M. L. Holt (1950). Tungsten(VI) Chloride (Tungsten Hexachloride). Inorganic Syntheses. 3. p. 163. doi:10.1002/9780470132340.ch44.
- ↑ J. C. Taylor; P. W. Wilson (1974). "The Structure of β-Tungsten Hexachloride by Powder Neutron and X-ray Diffraction". Acta Crystallographica B30 (5): 1216–1220. doi:10.1107/S0567740874004572..
- ↑ M. A. Umbreit, K. B. Sharpless (1990). "Deoxygenation of Epoxides with Lower Valent Tungsten Halides: trans-Cyclododecene". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV7P0121.; Collective Volume, 7, pp. 121
- ↑ Uhl, G.; Hey, E.; Becker, G.; Weller, F.; Dehnicke, K. (1983). "Über die Reaktion von 2,2-Dimethylpropylidinphosphan mit Wolframhexachlorid; die Kristallstrukturen von [(Cl3PO)WCL4(H9C4CCC4H9)] und [(H5C6)4As][WCL6]". Zeitschrift für Anorganische und Allgemeine Chemie 497 (2): 213–223. doi:10.1002/zaac.19834970221. http://nbn-resolving.de/urn:nbn:de:bsz:93-opus-57717.
- ↑ Lassner, Erik; Schubert, Wolf-Dieter (2012-12-06). Tungsten. Springer Science & Business Media. p. 145. ISBN 978-1-4615-4907-9.
- ↑ Meyer, R. J. (2013-09-03) (in de). Wolfram. Springer-Verlag. p. 207. ISBN 978-3-662-13401-6.
 |