Chemistry:Hexanitroethane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexanitroethane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C2N6O12 | |
| Molar mass | 300.0544 |
| Melting point | 135 °C (275 °F; 408 K) |
| Related compounds | |
Related compounds
|
Nitroethane Tetranitromethane Trinitromethane Hexanitrobenzene Octanitrocubane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
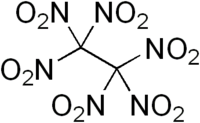
Hexanitroethane (HNE) is an organic compound with chemical formula C2N6O12 or (O2N)3C-C(NO2)3. It is a solid matter with a melting point of 135 °C.
Hexanitroethane is used in some pyrotechnic compositions as a nitrogen-rich oxidizer, e.g. in some decoy flare compositions and some propellants. Like hexanitrobenzene, HNE is investigated as a gas source for explosively pumped gas dynamic laser.
A composition of HNE as oxidizer with boron as fuel is being investigated as a new explosive.[1]
Preparation
The first synthesis was described by Wilhelm Will in 1914, using the reaction between the potassium salt of tetranitroethane with nitric acid.[2]
- C2(NO2)4K2 + 4 HNO3 → C2(NO2)6 + 2 KNO3 + 2 H2O
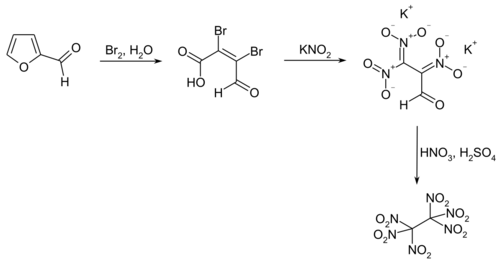
A practicable method for industrial use starts with furfural,[3] which first undergoes oxidative ring-opening by bromine to mucobromic acid.[4] In the following step, mucobromic acid is reacted with potassium nitrite at just below room temperature to form the dipotassium salt of 2,3,3-trinitropropanal. The final product is obtained by nitration with nitric acid and sulfuric acid at −60 °C.
Properties
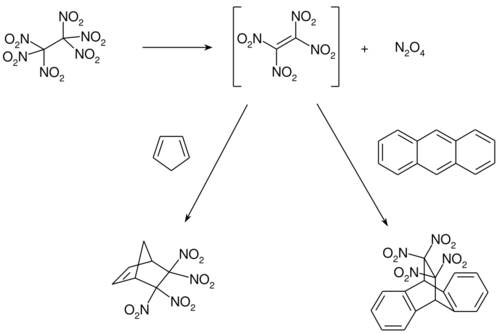
The thermal decomposition of hexanitroethane has been detected at 60 °C upwards in both the solid and solution phases.[5] Above 140 °C, this can occur explosively.[6] The decomposition is first order and is significantly faster in solution than in the solid. For the solid, the following reaction can be formulated:[5]
- C2(NO2)6 → 3 NO2 + NO + N2O + 2 CO2
For the decomposition is solution, tetranitroethylene is first formed and can be trapped and detected as a Diels–Alder adduct, for example with anthracene or cyclopentadiene.[7][8]
References
- ↑ Compatibility Testing of Hexanitroethane with Boron
- ↑ Will, W. (1914). "Über das Hexanitro-äthan" (in de). Berichte der Deutschen Chemischen Gesellschaft 47 (1): 961–965. doi:10.1002/cber.191404701154. https://zenodo.org/record/1426543.
- ↑ "Synthesis of hexanitroethane" US patent 3101379, issued 1961-01-04
- ↑ Taylor, G. (2015). "MUCOBROMIC ACID". Organic Syntheses: 11–12. doi:10.15227/orgsyn.000.0011. http://orgsyn.org/demo.aspx?prep=CV4P0688.
- ↑ 5.0 5.1 Marshall, Henry P.; Borgardt, Frank G.; Noble, Paul (1965). "Thermal Decomposition of Hexanitroethane 1a,b" (in en). The Journal of Physical Chemistry 69 (1): 25–29. doi:10.1021/j100885a007. ISSN 0022-3654.
- ↑ Bretherick, L. (18 March 2017). Bretherick's Handbook of Reactive Chemical hazards. Urben, P. G. (8th ed.). Amsterdam. pp. 240–241. ISBN 978-0-08-101059-4. OCLC 982005430.
- ↑ Griffin, T. Scott; Baum, Kurt (1980). "Tetranitroethylene. In situ formation and Diels-Alder reactions" (in en). The Journal of Organic Chemistry 45 (14): 2880–2883. doi:10.1021/jo01302a024. ISSN 0022-3263.
- ↑ Tzeng, Dongjaw; Baum, Kurt (1983). "Reactions of hexanitroethane with alcohols" (in en). The Journal of Organic Chemistry 48 (26): 5384–5385. doi:10.1021/jo00174a053. ISSN 0022-3263.
External links
 |