Chemistry:Indium perchlorate
From HandWiki
| |
| Names | |
|---|---|
| Other names
Indium triperchlorate, indium(III) perchlorate
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
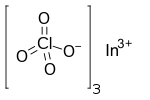
| In(ClO4)3 | |
| Molar mass | 413.17 |
| Appearance | colorless crystals |
| soluble | |
| Hazards | |
| Main hazards | Oxidizer |
| Related compounds | |
Other anions
|
indium nitrate, indium sulfate |
Other cations
|
aluminum perchlorate, gallium perchlorate, thallium perchlorate |
Related compounds
|
indium(I) perchlorate, indium chlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Indium perchlorate is the inorganic compound with the chemical formula In(ClO4)3.[1] The compound is an indium salt of perchloric acid.[2][3]
Synthesis
Dissolving indium hydroxide in perchloric acid:
- [math]\ce{ In(OH)3 + 3HClO4 -> In(ClO4)3 + 3H2O }[/math]
Physical properties
Indium(III) perchlorate forms colorless crystals. It is soluble in water and ethanol.
The compound forms a crystallohydrate In(ClO4)3•8H2O, that melts in its own crystallization water at 80 °C.[4]
The octahydrate is easily soluble in ethanol and acetic acid.
References
- ↑ Burgess, J. (31 October 2007) (in en). Inorganic Reaction Mechanisms: Volume 1. Royal Society of Chemistry. p. 110. ISBN 978-1-84755-648-6. https://books.google.com/books?id=ZnAoDwAAQBAJ&dq=indium(III)+perchlorate&pg=PA110. Retrieved 15 March 2023.
- ↑ "Indium Perchlorate" (in en). Russian Journal of Physical Chemistry (British Library Lending Division) 48, Part 3: 1611. 1974. https://books.google.com/books?id=E5gfAQAAMAAJ&q=indium(III)+perchlorate. Retrieved 15 March 2023.
- ↑ Eyring, Edward M.; Owen, Jeffrey D. (April 1970). "Kinetics of aqueous indium(III) perchlorate dimerization". The Journal of Physical Chemistry 74 (9): 1825–1828. doi:10.1021/j100704a001.
- ↑ "Indium(III) perchlorate hydrate". Sigma Aldrich. https://www.sigmaaldrich.com/RU/en/product/aldrich/401412.
 |

