Chemistry:Indium(III) fluoride
From HandWiki
| |

| |
| Names | |
|---|---|
| IUPAC name
Indium(III) fluoride
| |
| Other names
Indium trifluoride
| |
| Identifiers | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| InF3 | |
| Molar mass | 171.82 g/mol |
| Appearance | white solid |
| Density | 4.39 g/cm3 |
| Melting point | 1,172 °C (2,142 °F; 1,445 K)[1] |
| Structure | |
| Rhombohedral, hR24 | |
| R-3c, No. 167 | |
| Hazards[2] | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Flash point | non-flammable |
| Related compounds | |
Other anions
|
Indium(III) chloride Indium(III) bromide Indium(III) iodide |
Other cations
|
Aluminum fluoride Gallium(III) fluoride Thallium(I) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
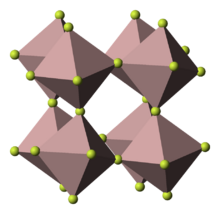
Indium(III) fluoride or indium trifluoride is the inorganic compound with the formula InF3. It is a white solid.
It has a rhombohedral crystal structure very similar to that of rhodium(III) fluoride. Each In center is octahedral. It is formed by the reaction of indium(III) oxide with hydrogen fluoride or hydrofluoric acid.[3]
Indium(III) fluoride is used in the synthesis of non-oxide glasses. It catalyzes the addition of trimethylsilyl cyanide (TMSCN) to aldehydes to form cyanohydrins.[2]
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 112, ISBN 0-8493-0594-2, https://books.google.com/books?id=lFjg0L-uOxoC&pg=PT1109, retrieved 2008-06-19
- ↑ 2.0 2.1 "435848 Indium(III) fluoride 99.9+ % trace metals basis". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/435848.
- ↑ Christoph Hebecker, R. Hoppe (1966). "Zur Kristallstrukur von Indiumtrifluorid und Thalliumtrifluorid (Crystal structure of In and Tl trifluorides)". Naturwissenschaften 53: 104. doi:10.1007/BF00601468.
 |

