Chemistry:Indium nitride
This article relies too much on references to primary sources. (January 2016) (Learn how and when to remove this template message) |
| |
| Names | |
|---|---|
| Other names
Indium(III) nitride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| InN | |
| Molar mass | 128.83 g/mol |
| Appearance | black powder |
| Density | 6.81 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) |
| hydrolysis | |
| Band gap | 0.65 eV (300 K) |
| Electron mobility | 3200 cm2/(V.s) (300 K) |
| Thermal conductivity | 45 W/(m⋅K) (300 K) |
Refractive index (nD)
|
2.9 |
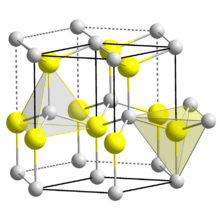
| Structure | |
| Wurtzite (hexagonal) | |
| C46v-P63mc | |
a = 354.5 pm, c = 570.3 pm [1]
| |
| Tetrahedral | |
| Hazards | |
| Main hazards | Irritant, hydrolysis to ammonia |
| Safety data sheet | External SDS |
| Related compounds | |
Other anions
|
Indium phosphide Indium arsenide Indium antimonide |
Other cations
|
Boron nitride Aluminium nitride Gallium nitride |
Related compounds
|
Indium gallium nitride Indium gallium aluminium nitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indium nitride (InN) is a small-bandgap semiconductor material, which has potential application in solar cells[2] and high speed electronics.[3][4]
The bandgap of InN has now been established as ~0.7 eV depending on temperature[5] (the obsolete value is 1.97 eV). The effective electron mass has been recently determined by high magnetic field measurements,[6][7] m* = 0.055 m0.
Alloyed with GaN, the ternary system InGaN has a direct bandgap span from the infrared (0.69 eV) to the ultraviolet (3.4 eV).
Currently there is research into developing solar cells using the nitride based semiconductors. Using one or more alloys of indium gallium nitride (InGaN), an optical match to the solar spectrum can be achieved. The bandgap of InN allows a wavelengths as long as 1900 nm to be utilized. However, there are many difficulties to be overcome if such solar cells are to become a commercial reality: p-type doping of InN and indium-rich InGaN is one of the biggest challenges. Heteroepitaxial growth of InN with other nitrides (GaN, AlN) has proved to be difficult.
Thin layers of InN can be grown using metalorganic chemical vapour deposition (MOCVD).[8]
Superconductivity
Thin polycrystalline films of indium nitride can be highly conductive and even superconductive at liquid helium temperatures. The superconducting transition temperature Tc depends on each sample's film structure and carrier density and varies from 0 K to about 3 K.[8][9] With magnesium doping, the Tc can be 3.97 K.[9] The superconductivity persists under high magnetic field (few teslas), that differs from superconductivity in In metal which is quenched by fields of only 0.03 tesla. Nevertheless, the superconductivity is attributed to metallic indium chains[8] or nanoclusters, where the small size increases the critical magnetic field according to the Ginzburg–Landau theory.[10]
See also
References
- ↑ Pichugin, I. G.; Tlachala, M. (1978). "Rentgenovsky analiz nitrida indiya" (in Russian). Izvestiya Akademii Nauk SSSR: Neorganicheskie Materialy 14 (1): 175–176.
- ↑ Nanishi, Y.; Araki, T.; Yamaguchi, T. (2010). "Molecular-beam epitaxy of InN". in Veal, T. D.; McConville, C. F.; Schaff, W. J.. Indium Nitride and Related Alloys. CRC Press. p. 31. ISBN 978-1-138-11672-6.
- ↑ Yim, J. W. L.; Wu, J. (2010). "Optical properties of InN and related alloys". in Veal, T. D.; McConville, C. F.; Schaff, W. J.. Indium Nitride and Related Alloys. CRC Press. p. 266. ISBN 978-1-138-11672-6.
- ↑ Christen, Jürgen; Gil, Bernard (2014). "Group III nitrides". Physica Status Solidi C 11 (2): 238. doi:10.1002/pssc.201470041. Bibcode: 2014PSSCR..11..238C.
- ↑ Monemar, B.; Paskov, P. P.; Kasic, A. (2005-07-01). "Optical properties of InN—the bandgap question" (in en). Superlattices and Microstructures 38 (1): 38–56. doi:10.1016/j.spmi.2005.04.006. ISSN 0749-6036. Bibcode: 2005SuMi...38...38M. https://www.sciencedirect.com/science/article/pii/S0749603605000558.
- ↑ Goiran, Michel; Millot, Marius; Poumirol, Jean-Marie; Gherasoiu, Iulian et al. (2010). "Electron cyclotron effective mass in indium nitride". Applied Physics Letters 96 (5): 052117. doi:10.1063/1.3304169. Bibcode: 2010ApPhL..96e2117G.
- ↑ Millot, Marius; Ubrig, Nicolas; Poumirol, Jean-Marie; Gherasoiu, Iulian et al. (2011). "Determination of effective mass in InN by high-field oscillatory magnetoabsorption spectroscopy". Physical Review B 83 (12). doi:10.1103/PhysRevB.83.125204. Bibcode: 2011PhRvB..83l5204M.
- ↑ 8.0 8.1 8.2 Inushima, Takashi (2006). "Electronic structure of superconducting InN". Science and Technology of Advanced Materials 7 (S1): S112–S116. doi:10.1016/j.stam.2006.06.004. Bibcode: 2006STAdM...7S.112I.
- ↑ 9.0 9.1 Tiras, E.; Gunes, M.; Balkan, N.; Airey, R. et al. (2009). "Superconductivity in heavily compensated Mg-doped InN". Applied Physics Letters 94 (14): 142108. doi:10.1063/1.3116120. Bibcode: 2009ApPhL..94n2108T. https://repository.essex.ac.uk/2240/1/063_1.pdf.
- ↑ Komissarova, T. A.; Parfeniev, R. V.; Ivanov, S. V. (2009). "Comment on 'Superconductivity in heavily compensated Mg-doped InN' [Appl. Phys. Lett. 94, 142108 (2009)]". Applied Physics Letters 95 (8): 086101. doi:10.1063/1.3212864. Bibcode: 2009ApPhL..95h6101K.
External links
- "InN – Indium nitride". Fiziko-tekhnichesky institut imeni A. F. Ioffe. n.d.. http://www.ioffe.ru/SVA/NSM/Semicond/InN/.
| NH3 | He(N2)11 | ||||||||||||||||
| Li3N | Be3N2 | BN | β-C3N4 g-C3N4 |
N2 | NxOy | NF3 | Ne | ||||||||||
| Na3N | Mg3N2 | AlN | Si3N4 | PN P3N5 |
SxNy SN S4N4 |
NCl3 | Ar | ||||||||||
| K3N | Ca3N2 | ScN | TiN | VN | CrN Cr2N |
MnxNy | FexNy | CoN | Ni3N | CuN | Zn3N2 | GaN | Ge3N4 | As | Se | NBr3 | Kr |
| Rb3N | Sr3N2 | YN | ZrN | NbN | β-Mo2N | Tc | Ru | Rh | PdN | Ag3N | CdN | InN | Sn | Sb | Te | NI3 | Xe |
| Cs3N | Ba3N2 | Hf3N4 | TaN | WN | Re | Os | Ir | Pt | Au | Hg3N2 | TlN | Pb | BiN | Po | At | Rn | |
| Fr3N | Ra3N | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | CeN | Pr | Nd | Pm | Sm | Eu | GdN | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UN | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |
