Chemistry:Iodosobenzene
| |
| Names | |
|---|---|
| Preferred IUPAC name
Iodosylbenzene[1] | |
| Other names
Iodosobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C 6H 5IO | |
| Molar mass | 220.01 g/mol |
| Appearance | colourless solid |
| Density | 1.229 g cm−3 |
| Melting point | 210 ˚C |
| poor | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H228, H271, H315, H319, H335 | |
| P210, P220, P240, P241, P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P280, P283, P302+352, P304+340, P305+351+338, P306+360, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P370+378, P371+380+375, P403+233, P405, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
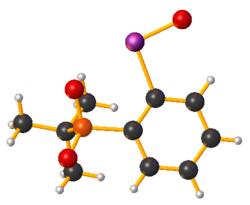
Iodosobenzene or iodosylbenzene is an organoiodine compound with the empirical formula C
6H
5IO. This colourless solid compound is used as an oxo transfer reagent in research laboratories examining organic and coordination chemistry.
Preparation and structure
Iodosobenzene is prepared from iodobenzene.[3] It is prepared by first oxidizing iodobenzene by peracetic acid. Hydrolysis of resulting diacetate affords "PhIO":[4]
- C
6H
5I + CH
3CO
3H + CH
3CO
2H → C
6H
5I(O
2CCH
3)
2 + H
2O - C
6H
5I(O
2CCH
3)
2 + H
2O → C
6H
5IO + 2 CH
3CO
2H
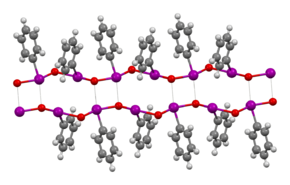
The structure of iodosobenzene has been verified by crystallographically.[5] Related derivatives are also oligomeric.[6] Its low solubility in most solvents and vibrational spectroscopy indicate that it is not molecular, but is polymeric, consisting of –I–O–I–O– chains.[7] The related diacetate, C
6H
5I(O
2CCH
3)
2, illustrates the ability of iodine(III) to adopt a T-shaped geometry without multiple bonds.[8]
Theoretical studies show that the bonding between the iodine and oxygen atoms in iodosobenzene represents a single dative I-O sigma bond, confirming the absence of the double I=O bond.[9]
A monomeric derivative iodosylbenzene is known in the form of 2-(tert-butylsulfonyl)iodosylbenzene, a yellow solid. C-I-O angle is 94.78°, C-I and I-O distances are 2.128 and 1.848 Å.[10]
Applications
Iodosobenzene has no commercial uses, but in the laboratory it is employed as an "oxo-transfer reagent." It epoxidizes certain alkenes and converts some metal complexes into the corresponding oxo derivatives. Although it is an oxidant, it is also mildly nucleophilic. These oxo-transfer reactions operate by the intermediacy of adducts PhI=O→M, which release PhI.[11]
A mixture of iodosobenzene and sodium azide in acetic acid converts alkenes to vicinal diazides:.[12][13]
- R
2C=CR
2 + 2 NaN
3 + PhIO + 2 AcOH → (N
3)R
2C–CR
2(N
3) + PhI + 2 AcONa + H
2O
Safety
This compound is explosive and should not be heated under vacuum.
See also
- Dess-Martin reagent
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 661. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ "Iodosylbenzene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/92125#section=Safety-and-Hazards.
- ↑ Conrad Willgerodt (1892). "Zur Kenntniss aromatischer Jodidchloride, des Jodoso- und Jodobenzols". Ber. 25 (2): 3494–3502. doi:10.1002/cber.189202502221. https://zenodo.org/record/1425674.
- ↑ H. Saltzman, J. G. Sharefkin (1963). "Iodosylbenzene". Organic Syntheses 43: 60. doi:10.15227/orgsyn.043.0060.
- ↑ Wegeberg, Christina; Frankær, Christian Grundahl; McKenzie, Christine J. (2016). "Reduction of hypervalent iodine by coordination to iron(III) and the crystal structures of PhIO and PhIO2". Dalton Transactions 45 (44): 17714–17722. doi:10.1039/C6DT02937J. PMID 27761533.
- ↑ Richter, Helen W.; Koser, Gerald F.; Incarvito, Christopher D.; Rheingold, Arnold L. (2007). "Preparation and Structure of a Solid-State Hypervalent-Iodine Polymer Containing Iodine and Oxygen Atoms in Fused 12-Atom Hexagonal Rings". Inorganic Chemistry 46 (14): 5555–5561. doi:10.1021/ic0701716. PMID 17569525.
- ↑ Hans Siebert; Monika Handrich (1976). "Schwingungsspektren und Struktur von Jodosyl- und Jodyl-Verbindungen". Z. anorg. allg. Chem. 426 (2): 173–183. doi:10.1002/zaac.19764260206.
- ↑ C. J. Carmalt, Claire J.; J. G. Crossley; J. G. Knight; P. Lightfoot; A. Martín; M. P. Muldowney; N. C. Norman; A. G. Orpen (1994). "An examination of the structures of iodosylbenzene (PhIO) and the related imido compound, PhINSO2-4-Me-C6H4, by X-ray powder diffraction and EXAFS (extended X-ray absorption fine structure) spectroscopy". J. Chem. Soc., Chem. Commun. (20): 2367–2368. doi:10.1039/C39940002367.
- ↑ Ivanov, A.; Popov, A.; Boldyrev, A.; Zhdankin, V. (2014). "The I=X (X = O,N,C) Double Bond in Hypervalent Iodine Compounds: Is it Real?". Angew. Chem. Int. Ed. 53 (36): 9617–9621. doi:10.1002/anie.201405142. PMID 25045143.
- ↑ MacIkenas, Dainius; Skrzypczak-Jankun, Ewa; Protasiewicz, John D. (2000). "Redirecting Secondary Bonds to Control Molecular and Crystal Properties of an Iodosyl- and an Iodylbenzene". Angewandte Chemie International Edition 39 (11): 2007–2010. doi:10.1002/1521-3773(20000602)39:11<2007::AID-ANIE2007>3.0.CO;2-Z. PMID 10941012.
- ↑ Lennartson, Anders; McKenzie, Christine J. (2012). "An Iron(III) Iodosylbenzene Complex: A Masked Non-Heme FeVO". Angewandte Chemie International Edition 51 (27): 6767–6770. doi:10.1002/anie.201202487. PMID 22639404.
- ↑ Robert M.Moriarty; Jaffar S.Khosrowshahi (1986). "A versatile synthesis of vicinal diazides using hypervalent iodine". Tetrahedron Lett. 27 (25): 2809–2812. doi:10.1016/S0040-4039(00)84648-1.
- ↑ March, J.; Smith, M. B. (2007). Advanced Organic Chemistry (6th ed.). New York: John Wiley & Sons. p. 1182. ISBN 978-0-471-72091-1.
 |