Chemistry:Isonicotinic acid
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyridine-4-carboxylic acid | |||
| Other names
Isonicotinic acid
4-Pyridinecarboxylic acid p-Pyridinecarboxylic acid 4-Picolinic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H5NO2 | |||
| Molar mass | 123.111 g·mol−1 | ||
| Appearance | White to off-white crystalline solid | ||
| Density | Solid | ||
| Melting point | 310 °C (590 °F; 583 K) (sublimes) | ||
| Hazards | |||
| Safety data sheet | http://datasheets.scbt.com/sc-250188.pdf | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
nicotinic acid, pyridine isoniazid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
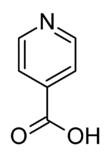
Isonicotinic acid or pyridine-4-carboxylic acid is an organic compound with the formula C5H4N(CO2H). It is a derivative of pyridine with a carboxylic acid substituent at the 4-position. It is an isomer of picolinic acid and nicotinic acid, which have the carboxyl group at the 2- and 3-position respectively compared to the 4-position for isonicotinic acid.
Derivatives
Isonicotinic acids is a term loosely used for derivatives of isonicotinic acid. Hydrazide derivatives include isoniazid, iproniazid, and nialamide. Amide and ester derivatives include ethionamide and dexamethasone isonicotinate.
See also
References
- ↑ Isonicotinic acid at chemicalland21.com
External links
- Isonicotinic+Acids at the US National Library of Medicine Medical Subject Headings (MeSH)
 |