Chemistry:Isophorone
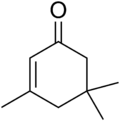
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,5,5-Trimethylcyclohex-2-en-1-one | |
| Other names
3,5,5-Trimethyl-2-cyclohexene-1-one
1,1,3-Trimethyl-3-cyclohexene-5-one Isoforone Isoacetophorone IP | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H14O | |
| Molar mass | 138.210 g·mol−1 |
| Appearance | Colorless to white liquid |
| Odor | Peppermint-like[2] |
| Density | 0.9255 g/cm3 |
| Melting point | −8.1 °C (17.4 °F; 265.0 K) |
| Boiling point | 215.32 °C (419.58 °F; 488.47 K) |
| 1.2 g/100 mL | |
| Solubility | ether, acetone, hexane, dichloromethane, benzene, toluene, alcohol |
| Vapor pressure | 0.3 mmHg (20°C)[2] |
Refractive index (nD)
|
1.4766 |
| Viscosity | 2.62 cP |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
43.4 kJ/mol |
| Hazards | |
| Flash point | 84 °C (183 °F; 357 K) |
| 460 °C (860 °F; 733 K) | |
| Explosive limits | 0.8–3.8%[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2280 mg/kg (rat, oral)[citation needed] 2330 mg/kg (rat, oral) 2690 mg/kg (mouse, oral)[3] |
LC50 (median concentration)
|
4600 ppm (guinea pig, 8 hr)[3] |
LCLo (lowest published)
|
885 ppm (rat, 6 hr) 1840 ppm (rat, 4 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 25 ppm (140 mg/m3)[2] |
REL (Recommended)
|
TWA 4 ppm (23 mg/m3)[2] |
IDLH (Immediate danger)
|
200 ppm[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially.[4]
Structure and reactivity
Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide.[5]
Isophorone is degraded by attack of hydroxyl radicals.[6]
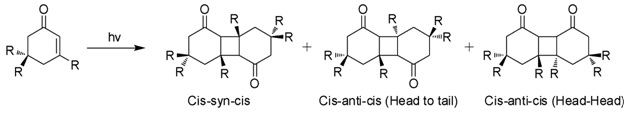
Photodimerization
When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium.[7]
Natural occurrence
Isophorone occurs naturally in cranberries.[8]
Synthesis
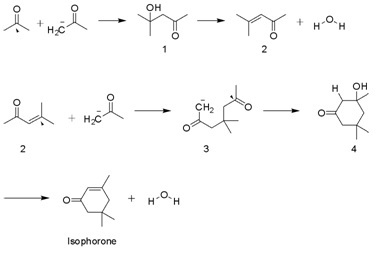
Isophorone is produced on a multi-thousand ton scale by the aldol condensation of acetone using KOH. Diacetone alcohol, mesityl oxide, and 3-hydroxy-3,5,5-trimethylcyclohexan-1-one are intermediates. A side product is beta-isophorone, where the C=C group is not conjugated with the ketone.[4]
Applications
The partly hydrogenated derivative trimethylcyclohexanone is used in production of polycarbonates. It condenses with phenol to give an analogue of bisphenol A. Polycarbonates produced by phosgenation of these two diols produces a polymer with improved thermal stability.[9] Trimethyladipic acid and 2,2,4-trimethylhexamethylenediamine are produced from trimethylcyclohexanone and trimethylcyclohexanol. They are used to make specialty polyamides. Hydrocyanation gives the nitrile followed by reductive amination gives isophorone diamine. This diamine is used to produce isophorone diisocyanate which has certain niche applications.[4]

Full hydrogenation gives 3,3,5-Trimethylcyclohexanol, a precursor to both sunscreens and chemical weapons.
Safety
The LD50 value of isophorone in rats and rabbits by oral exposure is around the 2.00 g/kg.[10] The safety aspects of isophorone have been subject to several studies.[11]
History
The use of isophorone as a solvent resulted from the search for ways to dispose of or recycle acetone, which is a waste product of phenol synthesis by the Hock method.[12]
See also
References
- ↑ Merck Index, 13th Edition, 5215.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 NIOSH Pocket Guide to Chemical Hazards. "#0355". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0355.html.
- ↑ 3.0 3.1 3.2 "Isophorone". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/78591.html.
- ↑ 4.0 4.1 4.2 Hardo Siegel; Manfred Eggersdorfer (2005). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 978-3-527-30673-2.
- ↑ Richard L. Wasson; Herbert O. House (1957). "Isophorone Oxide". Org. Synth. 37: 58. doi:10.15227/orgsyn.037.0058.
- ↑ "TOXNET". http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+78-59-1.
- ↑ Gonçalves, Huguette; Robinet, Germaine; Barthelat, Michèle; Lattes, Armand (1998-01-28). "Supramolecularity and Photodimerization of Isophorone: FTIR and Molecular Mechanics Studies" (in en). The Journal of Physical Chemistry A 102 (8): 1279–1287. doi:10.1021/jp9729270. Bibcode: 1998JPCA..102.1279G.
- ↑ "Isophorone". https://pubchem.ncbi.nlm.nih.gov/compound/Isophorone.
- ↑ Volker Serini (2000). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207. ISBN 978-3-527-30673-2.
- ↑ "Toxicity Effects". http://tools.niehs.nih.gov/cebs3/ntpviews/index.cfm?action=testarticle.toxicity&cas_number=389-08-2.
- ↑ W. Morton Grant, Joel S. Schuman M.D (11 February 2016). "Toxicology of the Eye: Effects on the Eyes and Visual System from Chemicals, Drugs, Metals and Minerals, Plants, Toxins, and Venoms; Also, Systemic Side Effects from Eye". Med (2-Volume Set) 4th Edition, Page 863.
- ↑ Isophorone history at Degussa
 |
