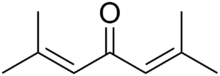
Chemistry:Phorone
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Dimethylhepta-2,5-dien-4-one | |
| Other names
Phorone
Diisopropylidene acetone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
| |
| |
| Properties | |
| ((CH 3) 2C=CH) 2C=O | |
| Molar mass | 138.210 g·mol−1 |
| Appearance | Yellow crystals |
| Odor | Geranium |
| Density | 0.885 g/cm3 |
| Melting point | 28 °C (82 °F; 301 K) |
| Boiling point | 198 to 199 °C (388 to 390 °F; 471 to 472 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| Flash point | 79 °C (174 °F; 352 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula C
9H
14O or ((CH
3)
2C=CH)
2C=O.
Preparation
It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle".[1] In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone".[2] On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid.[3][4]
- CaC
10H
14O
4 → C
9H
14O + CaCO
3
It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated.[5]

Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble.
Reactions
Phorone can condense with ammonia to form triacetone amine.
See also
References
- Merck Index, 11th Edition, 7307.
- ↑ Laurent, Auguste (1837). "Sur les acides pinique et sylvique, et sur le camphoryle" (in French). Annales de Chimie et de Physique. 2nd series 65: 324–332. https://babel.hathitrust.org/cgi/pt?id=hvd.hx3dx3;view=1up;seq=328.; see "Camphoryle", pp. 329–330.
- ↑ See:
- Gerhardt, Charles (1849) Comptes rendus des travaux de chimie (Paris, France: Masson, 1849), p. 385. (in French)
- Gerhardt; Liès-Bodart (1849). "Trockne Destillation des camphorsauren Kalks" (in German). Annalen der Chemie und Pharmacie 72 (3): 293–294. doi:10.1002/jlac.18490720327. https://babel.hathitrust.org/cgi/pt?id=mdp.39015026322084;view=1up;seq=673. From p. 293: "Dieses Oel, welches Gerhardt und Lies-Bodart mit dem Namen Phoron bezeichnen, … " (This oil, which Gerhardt and Liès-Bodart designate by the name "phorone", … )
- ↑ Watts, Henry, A Dictionary of Chemistry and the Allied Branches of Other Sciences (London, England: Longmans, Green, and Co., 1863), vol. 1, "Camphorone", p. 733.
- ↑ Kekulé, August (1866) (in German). Lehrbuch der organischen Chemie. 2nd vol.. Erlangen, (Germany): Ferdinand Enke. p. 463. https://books.google.com/books?id=XJlPAAAAYAAJ&pg=PA463.
- ↑ Hardo Siegel; Manfred Eggersdorfer (2005). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 978-3-527-30673-2.
External links
 |
