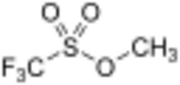
Chemistry:Methyl trifluoromethanesulfonate
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl trifluoromethanesulfonate | |
| Other names
Trifluoromethanesulfonic acid, methyl ester
Triflic acid, methyl ester, methyl triflate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2924 |
| |
| |
| Properties | |
| C2H3F3O3S | |
| Molar mass | 164.10 g·mol−1 |
| Appearance | Colourless Liquid |
| Density | 1.496 g/mL |
| Melting point | −64 °C (−83 °F; 209 K) |
| Boiling point | 100 °C (212 °F; 373 K) |
| Hydrolyzes | |
| Hazards[1] | |
| Main hazards | Corrosive |
| GHS Signal word | Danger |
| H226, H301, H311, H314, H330 | |
| P210, P233, P380, P303+361+353, P304+340+310, P305+351+338 | |
| Flash point | 38 °C (100 °F; 311 K) |
| Related compounds | |
Related compounds
|
Methyl fluorosulfonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF
3SO
2OCH
3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent.[2] The compound is closely related to methyl fluorosulfonate (FSO
2OCH
3). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence.[2][better source needed]
Synthesis
Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid.[3]
- CF
3SO
2OH + (CH
3O)
2SO
2 → CF
3SO
2OCH
3 + CH
3OSO
2OH
Reactivity
Hydrolysis
Upon contact with water, methyl triflate loses its methyl group, forming triflic acid and methanol:
- CF
3SO
2OCH
3 + H
2O → CF
3SO
2OH + CH
3OH
Methylation
One ranking of methylating agents is (CH
3)
3O+
> CF
3SO
2OCH
3 ≈ FSO
2OCH
3 > (CH
3)
2SO
4 > CH
3I.[3] Methyl triflate will alkylate many functional groups which are very poor nucleophiles such as aldehydes, amides, and nitriles. It does not methylate benzene or the bulky 2,6-di-tert-butylpyridine.[2] Its ability to methylate N-heterocycles is exploited in certain deprotection schemes.[4]
Cationic polymerization
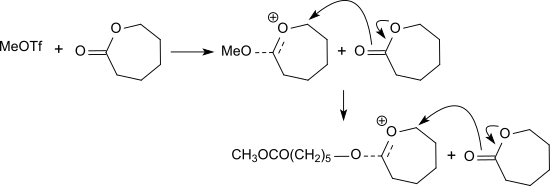
Methyl triflate initiates the living cationic polymerization of lactide[5] and other lactones including β-propiolactone, ε-caprolactone and glycolide.[6]
Cyclic carbonates like trimethylene carbonate and neopentylene carbonate (5,5-dimethyl-1,3-dioxan-2-one) can be polymerized to the corresponding polycarbonates.[7] 2-alkyl-2-oxazolines, for example 2-ethyl-2-oxazoline, are also polymerized to poly(2-alkyloxazoline)s.[8]
Applications
Radiochemistry
Carbon-11 methyl triflate ([11C]MeOTf[9]), or methyl triflate containing the carbon-11 isotope, is commonly used in radiochemistry to synthesize radioactively labeled compounds that can be traced in living organisms using positron emission tomography (PET). For example, [11C]MeOTf has been used extensively in the production of Pittsburgh Compound B, which first allowed β-amyloid plaques to be imaged in a living brain.
See also
- Triflate
- Magic methyl
References
- ↑ "Methyl trifluoromethanesulfonate". Sigma-Aldrich. https://www.sigmaaldrich.com/AU/en/product/ALDRICH/164283.
- ↑ 2.0 2.1 2.2 Roger W. Alder; Justin G. E. Phillips; Lijun Huang; Xuefei Huang (2005). "Methyltrifluoromethanesulfonate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm266m.pub2. ISBN 0471936235.
- ↑ 3.0 3.1 Stang, Peter J.; Hanack, Michael; Subramanian, L. R. (1982). "Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry". Synthesis 1982 (2): 85–126. doi:10.1055/s-1982-29711. ISSN 0039-7881.
- ↑ Albert I. Meyers; Mark E. Flanagan (1998). "2,2′-Dimethoxy-6-formylbiphenyl". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV9P0258.; Collective Volume, 9, pp. 258
- ↑ Rangel, Irma; Ricard, Michèle; Ricard, Alain (1994). "Polymerization of L-lactide and ε-caprolactone in the presence of methyl trifluoromethanesulfonate". Macromolecular Chemistry and Physics 195 (9): 3095–3101. doi:10.1002/macp.1994.021950908.
- ↑ Jonté, J. Michael; Dunsing, Ruth; Kricheldorf, Hans R. (1985). "Polylactones. 4. Cationic Polymerization of Lactones by Means of Alkylsulfonates" (in en). Journal of Macromolecular Science: Part A - Chemistry 22 (4): 495–514. doi:10.1080/00222338508056616. ISSN 0022-233X.
- ↑ Kricheldorf, Hans R.; Weegen-Schulz, Bettina; Jenssen, Jörg (1998). "Cationic polymerization of aliphatic cyclocarbonates" (in en). Macromolecular Symposia 132 (1): 421–430. doi:10.1002/masy.19981320139.
- ↑ Glassner, Mathias; D’hooge, Dagmar R.; Young Park, Jin; Van Steenberge, Paul H.M.; Monnery, Bryn D.; Reyniers, Marie-Françoise; Hoogenboom, Richard (2015). "Systematic investigation of alkyl sulfonate initiators for the cationic ring-opening polymerization of 2-oxazolines revealing optimal combinations of monomers and initiators" (in en). European Polymer Journal 65: 298–304. doi:10.1016/j.eurpolymj.2015.01.019. https://biblio.ugent.be/publication/5924229.
- ↑ Jewett, D. M. (1992). "A simple synthesis of [11Cmethyl triflate"]. International Journal of Radiation Applications and Instrumentation, Part A 43 (11): 1383–1385. doi:10.1016/0883-2889(92)90012-4. ISSN 0883-2889. PMID 1333459. https://pubmed.ncbi.nlm.nih.gov/1333459/.
 |