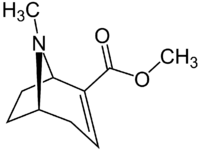
Chemistry:Methylecgonidine
| |
| Names | |
|---|---|
| IUPAC name
Methyl trop-2-ene-2β-carboxylate
| |
| Systematic IUPAC name
Methyl (1R,5S)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene-2-carboxylate | |
| Other names
Anhydromethylecgonine
Anhydroecgonine methyl ester | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H15NO2 | |
| Molar mass | 181.235 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester; AEME) is a chemical intermediate derived from ecgonine or cocaine.
Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form methylecgonidine as a metabolite.[1] Methylecgonidine has a relatively short half-life of 18–21 minutes, after which it is metabolised to ecgonidine, meaning that the relative concentrations of the two compounds can be used to estimate how recently crack cocaine has been smoked. Methylecgonidine has been shown to be specifically more harmful to the body than other byproducts of cocaine; for example to the heart,[2] lungs[3] & liver.[4] The toxicity is due to a partial agonist effect at M1 and M3 muscarinic receptors, leading to DNA fragmentation and neuronal death by apoptosis.[5]
AEME is also used in scientific research for the manufacture of phenyltropane analogues such as troparil, dichloropane, iometopane, and CFT. Methylecgonidine could also theoretically be used to produce cocaine and so may be a controlled substance in some countries.
Synthesis
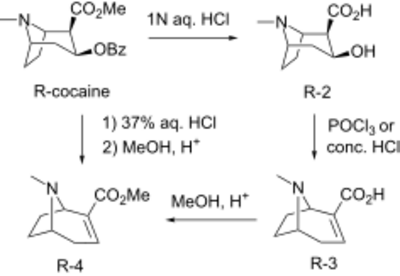
Methylecgonidine can be synthesized non pyrolytically from cocaine via hydrolysis/dehydration[6] followed by esterification with methanol.[7][8]
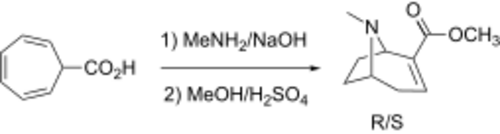
The scheme by Kline[9] is based on the reaction of 2,4,6-cycloheptatriene-7-carboxylic acid with methylamine. This is a modified version of U.S. Patent 2,783,235 by Grundmann and Ottmann. In the accompanying patent U.S. Patent 2,783,236 these same authors react their methylecgonidine with two equivalents of PhLi to form a tertiary alcohol by "hard" addition to the ester and not "soft" Michael addition. However, the product is only one tenth the potency of atropine. The methyl 2,4,6-cycloheptatriene-1-carboxylate can be made synthetically.[10][11]
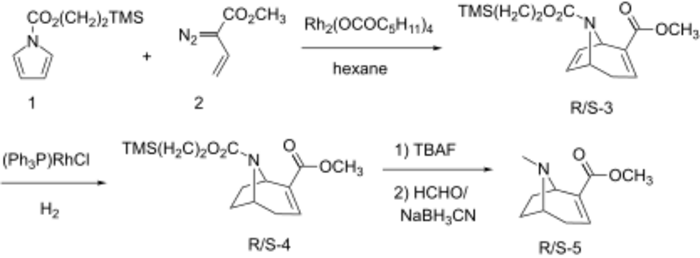
Davies et al. synthesized (R/S)-methylecgonidine by a tandem cyclopropanation/Cope rearrangement.[13][14] Thus, reaction of methyldiazobutenoate (2) with 5 equiv of N-((2-(TMS)ethoxy)carbonyl)pyrrole (1) in the presence of rhodium(II) hexanoate/hexane gave the [3.2.1]-azabicyclic system (R/S)-8 in 62% yield. The unsubstituted double bond was selectively reduced using Wilkinson catalyst to provide N-protected anhydroecgonine methyl ester ((R/S)-4). Following deprotection of N8 nitrogen with TBAF and reductive methylation with formaldehyde and sodium cyanoborohydride, (R/S)-5 was obtained in overall good yield.
See also
References
- ↑ "Pharmacokinetics and pharmacodynamics of methylecgonidine, a crack cocaine pyrolyzate". The Journal of Pharmacology and Experimental Therapeutics 307 (3): 1179–87. December 2003. doi:10.1124/jpet.103.055434. PMID 14561847.
- ↑ Pharmacokinetics and Pharmacodynamics of Methylecgonidine, a Crack Cocaine Pyrolyzate - Scheidweiler et al. 307 (3): 1179 Figure IG6 - Journal of Pharmacology and Experimental Therapeutics
- ↑ "Evidence for cocaine and methylecgonidine stimulation of M(2) muscarinic receptors in cultured human embryonic lung cells". British Journal of Pharmacology 132 (2): 451–60. January 2001. doi:10.1038/sj.bjp.0703819. PMID 11159694.
- ↑ "Studies on hydrolytic and oxidative metabolic pathways of anhydroecgonine methyl ester (methylecgonidine) using microsomal preparations from rat organs". Chemical Research in Toxicology 15 (12): 1543–8. December 2002. doi:10.1021/tx0255828. PMID 12482236.
- ↑ "M1 and M3 muscarinic receptors may play a role in the neurotoxicity of anhydroecgonine methyl ester, a cocaine pyrolysis product". Scientific Reports 5: 17555. December 2015. doi:10.1038/srep17555. PMID 26626425. Bibcode: 2015NatSR...517555G.
- ↑ "Generation of polyclonal catalytic antibodies against cocaine using transition state analogs of cocaine conjugated to diphtheria toxoid". Chemical & Pharmaceutical Bulletin 43 (11): 1902–11. November 1995. doi:10.1021/ja01502a049. PMID 8575031.
- ↑ "Some properties of the ecgonines and their esters II. The structural formulae of the ecgonines and ecgonidine.". Recueil des Travaux Chimiques des Pays-Bas 56 (2): 186–97, 198–201. 1937. doi:10.1002/recl.19370560215.
- ↑ "Isolation of Ecgonidine Methyl Ester from Coca Seeds 1". J. Am. Chem. Soc. 63 (9): 2444–2446. 1941. doi:10.1021/ja01854a038.
- ↑ "Synthesis of 3-arylecgonine analogues as inhibitors of cocaine binding and dopamine uptake". Journal of Medicinal Chemistry 33 (7): 2024–7. July 1990. doi:10.1021/jm00169a036. PMID 2362282.
- ↑ "Methyl 2,4,6-cycloheptatriene-1-carboxylate - C9H10O2, density, melting point, boiling point, structural formula, synthesis". http://www.chemsynthesis.com/base/chemical-structure-15475.html.
- ↑ "Transition-metal-catalyzed reactions of diazo compounds. 2. Addition to aromatic molecules: Catalysis of Buchner's synthesis of cycloheptatrienes". The Journal of Organic Chemistry 46 (5): 873–876. 1981. doi:10.1021/jo00318a010. https://orbi.uliege.be/handle/2268/237697.
- ↑ "Enantioselective synthesis of tropanes by reaction of rhodium-stabilized vinylcarbenoids with pyrroles". Tetrahedron Letters 33 (46): 6935–6938. 1992. doi:10.1016/S0040-4039(00)60899-7. ISSN 0040-4039.
- ↑ Davies, H. M. L.; Saikali, E.; Young, W. B. (1991). "Synthesis of (.+-.)-ferruginine and (.+-.)-anhydroecgonine methyl-ester by a tandem cyclopropanation/Cope rearrangement". J. Org. Chem. 56 (19): 5696–5700. doi:10.1021/jo00019a044.
- ↑ "Novel entry to the tropane system by reaction of rhodium (II) acetate stabilized vinylcarbenoids with pyrroles.". Tetrahedron Letters 30 (35): 4653–6. January 1989. doi:10.1016/S0040-4039(01)80766-8.
 |
