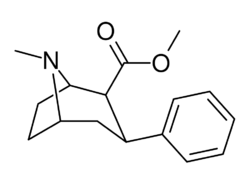
Chemistry:Troparil
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H21NO2 |
| Molar mass | 259.349 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 190 to 191 °C (374 to 376 °F) |
| |
| |
| | |
Troparil (also known as (–)-2β-Carbomethoxy-3β-phenyltropane, WIN 35,065-2, or β-CPT) is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor,[1] but is less potent as a serotonin reuptake inhibitor,[2] and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine.[3] The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
Background
The first known published synthesis of troparil and the related compound WIN 35428 is by Clarke and co-workers during the 1970s.[4][5] Apparently, it was their intention to separate the stimulant actions of cocaine from its toxicity and dependence liability. Troparil is the only regular phenyltropane having a NET affinity that exceeds the DAT affinity.[citation needed]
Application
Phenyltropanes are likely to have less abuse and dependency compared with cocaine.[6][7]
Troparil is used in scientific research into the dopamine reuptake transporter. 3H-radiolabelled forms of troparil have been used in humans and animals to map the distribution of dopamine transporters in the brain.[8][9] It is also used for animal research into stimulant drugs as an alternative to cocaine which produces similar effects,[10] but avoids the stringent licensing requirements for the use of cocaine itself.
Troparil has similar effects to cocaine in animal studies,[11][12] but recreational use of this compound to date has proven extremely rare. Despite being easily made by the reaction of methylecgonidine with phenylmagnesium bromide,[5][13] the relative scarcity of methylecgonidine and the demanding reaction conditions required for the synthesis[14][15] put production of this compound beyond the capacity of most illicit drug manufacturers, and legitimate supplies of troparil are available only in very small quantities for a very high price.
Legality
The legal status of troparil is unclear, but it may be considered a controlled substance analog of cocaine in the United States on the grounds of its related chemical structure. The legal status of troparil and many other cocaine analogs in Canada, is dependent on if ecgonine, coca, or cocaine were derivatives of the compound, according to the wording on the entry of coca in Schedule 1 of the Controlled Drugs and Substances Act.[16]
See also
- List of phenyltropanes
- List of cocaine analogues
- Amfonelic acid
References
- ↑ "Dopamine transporter ligands: recent developments and therapeutic potential". Current Topics in Medicinal Chemistry 6 (17): 1825–43. 2006. doi:10.2174/156802606778249775. PMID 17017960.
- ↑ "Cocaine and 3 beta-(4'-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues. New high-affinity and selective compounds for the dopamine transporter". Journal of Medicinal Chemistry 38 (2): 379–88. January 1995. doi:10.1021/jm00002a020. PMID 7830281.
- ↑ "Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options". American Journal of Cardiovascular Drugs 9 (3): 177–96. 2009. doi:10.1007/bf03256574. PMID 19463023.
- ↑ U.S. Patent 3,813,404
- ↑ 5.0 5.1 "Compounds affecting the central nervous system. 4. 3 Beta-phenyltropane-2-carboxylic esters and analogs". Journal of Medicinal Chemistry 16 (11): 1260–7. November 1973. doi:10.1021/jm00269a600. PMID 4747968.
- ↑ "A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants". Neuropsychopharmacology 31 (2): 351–62. February 2006. doi:10.1038/sj.npp.1300795. PMID 15957006.
- ↑ "Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys". Pharmacology, Biochemistry, and Behavior 86 (1): 45–54. January 2007. doi:10.1016/j.pbb.2006.12.006. PMID 17258302.
- ↑ "[3HWIN 35,065-2: a ligand for cocaine receptors in striatum"]. Journal of Neurochemistry 55 (5): 1556–62. November 1990. doi:10.1111/j.1471-4159.1990.tb04938.x. PMID 2120386. https://zenodo.org/record/1230647.
- ↑ "Cocaine receptors: in vivo labeling with 3H-(-)cocaine, 3H-WIN 35,065-2, and 3H-WIN 35,428". Synapse 4 (4): 390–2. 1989. doi:10.1002/syn.890040415. PMID 2603151. https://zenodo.org/record/1229373.
- ↑ "[Pharmacology of troparil]". Farmakologiia I Toksikologiia 48 (1): 15–9. 1985. PMID 3838516.
- ↑ "Potent substituted-3 beta-phenyltropane analogs of cocaine have cocaine-like discriminative stimulus effects". Drug and Alcohol Dependence 29 (2): 145–51. December 1991. doi:10.1016/0376-8716(91)90043-X. PMID 1797525.
- ↑ "Synthesis, dopamine transporter affinity, dopamine uptake inhibition, and locomotor stimulant activity of 2-substituted 3 beta-phenyltropane derivatives". Journal of Medicinal Chemistry 40 (6): 858–63. March 1997. doi:10.1021/jm960739c. PMID 9083474.
- ↑ "Synthesis of 3-arylecgonine analogues as inhibitors of cocaine binding and dopamine uptake". Journal of Medicinal Chemistry 33 (7): 2024–7. July 1990. doi:10.1021/jm00169a036. PMID 2362282.
- ↑ "Stereoselective Synthesis of 2β-Carbomethoxy-3β-Phenyltropane Derivatives. Enhanced Stereoselectivity Observed for the Conjugate Addition Reaction of Phenylmagnesium Bromide Derivatives with Anhydro Dichloromethane". Journal of Heterocyclic Chemistry 33 (6): 2037–2039. 1996. doi:10.1002/jhet.5570330676.
- ↑ "Synthesis and receptor binding of N-substituted tropane derivatives. High-affinity ligands for the cocaine receptor". Journal of Medicinal Chemistry 34 (5): 1728–31. May 1991. doi:10.1021/jm00109a029. PMID 2033595.
- ↑ "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". Department of Justice. Government of Canada. http://laws-lois.justice.gc.ca/eng/acts/c-38.8/page-24.html#h-28.
 |
