Chemistry:Arecoline
Arecoline is a cholinergic agent, stimulant, and naturally occurring alkaloid found in areca (betel) nuts of the areca palm (Areca catechu) found in South and Southeast Asia.[1][2] Its effects, depending on the dose, include stimulation, alertness, increased concentration, cognitive enhancement, elation, euphoria, pro-sexual effects, relaxation, reduced anxiety, and sedation, as well as addiction and withdrawal symptoms upon discontinuation.[1] Its effects are described as subtle and it has been likened to a strong cup of coffee.[2] There are also active constituents of areca nuts, but arecoline is the key active component, with a percentage of ~0.3 to 0.6%.[1] Areca nuts are administered by chewing for 5 to 20 minutes without swallowing.[1]
Side effects of arecoline include hypersalivation, hypotension, vertigo, miosis, tremor, and bradycardia, among others.[1] Other adverse effects can include extrapyramidal syndrome and seizures.[1] Addiction and dependence can occur, with withdrawal symptoms including mood swings, anxiety, irritability, and insomnia.[1] Rarely, psychosis can occur during withdrawal in heavy users.[1] Areca nut use, the primary method of consuming arecoline, is highly associated with oral disease and oral cancer.[2] Overdose of arecoline can be treated with antimuscarinic drugs like atropine or scopolamine.[1]
The drug acts as a non-selective partial agonist of muscarinic and nicotinic acetylcholine receptors.[1] The major metabolite of arecoline, arecaidine, is a GABA reuptake inhibitor.[1] The subjective effects of arecoline appear to be mediated by muscarinic acetylcholine receptors and not by nicotinic acetylcholine receptors based on animal studies.[1][3] However, its mechanism of action is still yet to be fully understood and other activities have also been described.[2][4] The stimulant and addictive effects of arecoline are thought to be due to increased dopaminergic neurotransmission in the mesolimbic pathway of the brain.[5][2][1] In terms of chemical structure, arecoline is closely related to nicotinic acid.[1]
The use of arecoline, in the form of areca nuts, dates back several thousand years, including in Thailand, China, and Polynesia.[1] Arecoline was first isolated in 1888 and its synthesis was first proposed in 1891, with its chemical structure confirmed in 1907.[1] Arecoline, in the form of areca nuts, is used by more than 600 million people worldwide (~10–20% of the global population), and is the fourth most commonly used psychoactive drug in the world after alcohol, nicotine, and caffeine.[1][2] Despite globalization, arecoline is unusual among recreational drugs in that its use is still predominantly confined to Asia, though its use has been increasing and spreading in part due to the Internet.[1][2] Chewing areca nuts is said to be as familiar to various Asian peoples as chewing gum is to Americans.[1] The countries in which areca nut production are highest include India, China, Myanmar, Malaysia, Indonesia, and Bangladesh.[1] Arecoline and areca nut sale and consumption are not generally controlled throughout the world, with a few exceptions.[1]
Uses
Recreational
In many Asian cultures, the areca nut is chewed along with betel leaf to obtain a stimulating effect.[6]
Medical
Arecoline has been used medicinally as an antihelmintic (a drug against parasitic worms).[7] It paralyzes tapeworms.[8]
Traditional medicine
Arecoline has been used in traditional medicine in Asia for thousands of years.[1]
Toxicity
The -1">50 of arecoline is 100 mg/kg, when administered subcutaneously in mice.[9] The minimum lethal dose (MLD) values of arecoline in mice, dogs, and horses is 100 mg/kg, 5 mg/kg and 1.4 mg/kg respectively.[8]
It causes oral submucous fibrosis by stimulating collagen, interleukin 6, keratinocyte growth factor-1, IGF-1, cystatin C, tissue inhibitor of matrix metalloproteinases in the mouth.[citation needed]
Current science is confident that areca nut chewing is carcinogenic. Research suggests this is probably at least partly because of arecoline itself, although it could also be from the other constituents of the nut as well, some of which are precursors to nitrosamines that form in the mouth during chewing. Section 5.5 Evaluation on page 238 of IARC Monograph 85-6 states the following:[10]
- [...]
- There is sufficient evidence in humans for the carcinogenicity of betel quid without tobacco. Betel quid without tobacco causes oral cancer.
- There is sufficient evidence in experimental animals for the carcinogenicity of betel quid without tobacco.
- There is sufficient evidence in experimental animals for the carcinogenicity of betel quid with tobacco.
- There is sufficient evidence in experimental animals for the carcinogenicity of areca nut.
- There is sufficient evidence in experimental animals for the carcinogenicity of areca nut with tobacco.
- There is limited evidence in experimental animals for the carcinogenicity of arecoline.
- There is inadequate evidence in experimental animals for the carcinogenicity of arecaidine.
- [...]
The toxicity of arecoline can be partially mitigated by vitamins C and E in mice.[11]
Mechanisms of toxicity
Arecoline is "obviously cytotoxic" to cultures of hepatocytes, bone marrow cells, lymphocytes, neuronal cell, myoblasts and endothelial cells.[8]
Arecoline generates excessive reactive oxygen species (ROS) in a number of cell types, including oral epithelial cells and neuronal cells. In adult mice, arecoline is toxic to the testes and liver via ROS generation.[8]
Arecoline is also genotoxic, being able to induce DNA damage and mutation in several cell cultures.[8] Mice chronically exposed to arecoline show relaxation of their chromatin structure.[12]
Pharmacology
Pharmacodynamics
Arecoline is the primary active ingredient responsible for the central nervous system effects of the areca nut. Arecoline has been compared to nicotine; however, nicotine agonizes nicotinic acetylcholine receptors, whereas arecoline is primarily a partial agonist of muscarinic acetylcholine receptors,[13][14] leading to its parasympathetic effects. In frogs, arecoline also acts as an antagonist (or very weak partial agonist) at α4 and α6-containing nicotinic acetylcholine receptors and as a silent antagonist at α7 nicotinic receptors, which may account for its anti-inflammatory activity.[15] Arecoline also inhibits AMPK through generation of ROS in several types of cells.[16]
AN (Areca Nut) is a vasodilator mainly due to the presence of arecoline. It also has anti-thrombosis and anti-atherogenic effects by increasing plasma nitric oxide, eNos, and mRNA expression and decreasing IL-8 along with other downregulations.[8] It increases the level of testosterone by stimulating Leydig's cells as well as levels of FSH and LH.[17][18] It also activates HPA axis and stimulates CRH release. It prevents the dysfunction of B cells of the pancreas from high fructose intake.[8] Arecoline has the ability to stimulate the digestive system through the activation of muscarinic receptors. Areca nut water extract could increase the contractions of gastric smooth muscle and muscle strips of the duodenum, ileum, and colon significantly. This activity could be caused by arecoline.[8]
Pharmacokinetics
Arecoline is metabolized by both kidneys and liver.[19] Currently, 11 metabolites of arecoline are documented among which N-methylnipecotic acid was found to be a major metabolite of both arecoline and arecaidine.[20] Lime, which is traditionally mixed to crushed areca nuts prior to consumption, is said to hydrolyse almost all arecoline to arecaidine, a GABA reuptake inhibitor.[21] Arecaidine is also formed during liver metabolism of arecoline in rats.[20]
Arecoline is very efficiently absorbed through oral musoca, with 85% bioavailbility. Maximum plasma concentration is reached within 3 minutes.[22]
Orally ingested arecoline is extensively metabolized in rats, with the vast majority of the dose being converted to arecaidine and arecoline N-oxide.[23]
Chemistry
Arecoline is a colorless odorless oily liquid.[1] It is a base, and its conjugate acid has a pKa ~ 6.8.[9] Arecoline is volatile in steam, miscible with most organic solvents and water, but extractable from water by ether in presence of dissolved salts. Being basic, arecoline forms salts with acids. The salts are crystalline, but usually deliquescent: the hydrochloride, arecoline•HCl, forms needles, m.p. 158 °C;[9] the hydrobromide, arecoline•HBr, forms slender prisms, mp. 177–179 °C from hot methanol; the aurichloride, arecoline•HAuCl4, is an oil, but the platinichloride, arecoline2•H2PtCl6, mp. 176 °C, crystallizes from water in orange-red rhombohedrons. The methiodide forms glancing prisms, mp. 173–174 °C.
Synthesis
Although an older method was described in the patent literature,[24] this is less attractive than the modern methods.
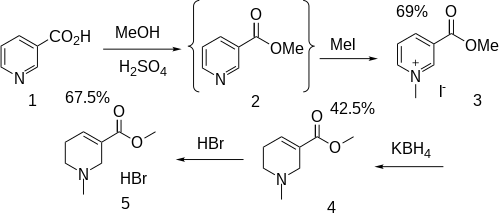
Fischer esterification of nicotinic acid (niacin) (1) gives methyl nicotinate (2). Alkylation with methyl iodide then gives 3-methoxycarbonyl-1-methylpyridinium iodide (3). Hydride reduction with an agent such as potassium borohydride thus gives the tetrahydropyridine (4). Salt formation with HBr completes the synthesis (5).

A double Mannich reaction between methylamine (1), acetaldehyde (2) and formaldehyde (3) in the presence of hydroxylamine hydrochloride is supposed to have delivered 1-methyl-1,2,5,6-tetrahydropyridine-3-carbaldehyde oxime hydrochloride (4) as the product. Dehydration of the aldoxime to the nitrile occurs upon treatment with acetic anhydride giving 3-cyano-1-methyl-1,2,5,6-tetrahydropyridine (5). Functional group interconversion of the nitrile to the methyl carboxylate ester then occurs upon acid-catalyzed treatment in methanol, and then conversion to the HBr salt completes the synthesis.
Chemical precursor
Arecoline is used in the synthesis of a variety of other drugs, including paroxetine,[28][29] femoxetine, nocaine, piquindone,[30] PC10058081 (epiboxidine type), FT-0731096 [114724-56-0], piper-brasofensine[31] piper-Tesofensine[32] and BRN 0023391 [102206-67-7].
Analogues
Analogues of and related compounds to arecoline include arecaidine, guavacoline, guvacine, nipecotic acid, nicotinic acid, muscarine, SKF-89976A, tiagabine, and CI-966. Xanomeline, the anti-schizophrenia component in the approved drug xanomeline/trospium chloride, also has structural similarities to arecoline.[33]
Research
Owing to its muscarinic and nicotinic agonist properties, arecoline has shown improvement in the learning ability of healthy volunteers. Since one of the hallmarks of Alzheimer's disease is a cognitive decline, arecoline was suggested as a treatment to slow down this process. Arecoline administered intravenously did indeed show modest verbal and spatial memory improvement in Alzheimer's patients,[34] though due to arecoline's possible carcinogenic properties (see § Toxicity), it is not the first drug of choice for this degenerative disease.[34]
Anecdotal reports indicate that it has a short-lived effect against schizophrenia. Among male schizophrenia patients, higher areca nut consumption is associated with weaker symptoms. It inspired the development of xanomeline.[33] It enhances learning and memory in rodents.[8]
Veterinary use
In 2012, Chinese Ministry of Agriculture listed arecoline hydrobromide as an abolished veterinary drug and stopped its production and use.[35]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 "DARK Classics in Chemical Neuroscience: Arecoline". ACS Chemical Neuroscience 10 (5): 2176–2185. May 2019. doi:10.1021/acschemneuro.8b00711. PMID 30664352. https://bitnest.netfirms.com/external/10.1021/acschemneuro.8b00711.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Betel quid: New insights into an ancient addiction". Addict Biol 27 (5). September 2022. doi:10.1111/adb.13223. PMID 36001424.
- ↑ "Discriminative stimulus properties of arecoline: a new approach for studying central muscarinic receptors". Psychopharmacology (Berl) 75 (4): 383–387. 1981. doi:10.1007/BF00435858. PMID 6803285.
- ↑ "Cracking the Betel Nut: Cholinergic Activity of Areca Alkaloids and Related Compounds". Nicotine Tob Res 21 (6): 805–812. May 2019. doi:10.1093/ntr/ntx187. PMID 29059390.
- ↑ "Intoxication and substance use disorder to Areca catechu nut containing betel quid: A review of epidemiological evidence, pharmacological basis and social factors influencing quitting strategies". Drug Alcohol Depend 179: 187–197. October 2017. doi:10.1016/j.drugalcdep.2017.06.039. PMID 28787696.
- ↑ "Epidemiology of betel quid usage". Annals of the Academy of Medicine, Singapore 33 (4 Suppl): 31–36. July 2004. doi:10.47102/annals-acadmedsg.V33N4p31S. PMID 15389304. http://www.annals.edu.sg/pdf200409/V33N4p31S.pdf.
- ↑ "Oral submucous fibrosis in a 12-year-old Bangladeshi boy: a case report and review of literature". International Journal of Paediatric Dentistry 12 (4): 271–276. July 2002. doi:10.1046/j.1365-263X.2002.00373.x. PMID 12121538.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 "The pharmacology, toxicology and potential applications of arecoline: a review". Pharmaceutical Biology 54 (11): 2753–2760. November 2016. doi:10.3109/13880209.2016.1160251. PMID 27046150.
- ↑ 9.0 9.1 9.2 The Merck index: an encyclopedia of chemicals, drugs, and biologicals (10th ed.). Rahway, N.J., U.S.A.: Merck & Co.. 1983. p. 113. ISBN 978-0-911910-27-8.
- ↑ International Agency for Research on Cancer (2005). Betel-quid and areca-nut chewing. IARC Monograph 85-6. IARC. ISBN 978-92-832-1285-0. http://monographs.iarc.fr/ENG/Monographs/vol85/mono85-6.pdf.
- ↑ "The hepatotoxicity and testicular toxicity induced by arecoline in mice and protective effects of vitamins C and e". The Korean Journal of Physiology & Pharmacology 18 (2): 143–148. April 2014. doi:10.4196/kjpp.2014.18.2.143. PMID 24757376.
- ↑ "Arecoline-induced changes of poly-ADP-ribosylation of cellular proteins and its influence on chromatin organization". Cancer Letters 139 (1): 59–65. May 1999. doi:10.1016/S0304-3835(99)00008-7. PMID 10408909.
- ↑ "Differential receptor occupancy requirements for muscarinic cholinergic stimulation of inositol lipid hydrolysis in brain and in neuroblastomas". Molecular Pharmacology 32 (1): 81–90. July 1987. doi:10.1016/S0026-895X(25)13765-6. PMID 3600615.
- ↑ "Pharmacologic comparison of selected agonists for the M1 muscarinic receptor in transfected murine fibroblast cells (B82)". The Journal of Pharmacology and Experimental Therapeutics 256 (2): 689–694. February 1991. doi:10.1016/S0022-3565(25)22996-2. PMID 1704434.
- ↑ "Nicotinic Activity of Arecoline, the Psychoactive Element of "Betel Nuts", Suggests a Basis for Habitual Use and Anti-Inflammatory Activity". PLOS ONE 10 (10). 2015. doi:10.1371/journal.pone.0140907. PMID 26488401. Bibcode: 2015PLoSO..1040907P.
- ↑ "Arecoline-mediated inhibition of AMP-activated protein kinase through reactive oxygen species is required for apoptosis induction". Oral Oncology 47 (5): 345–351. May 2011. doi:10.1016/j.oraloncology.2011.02.014. PMID 21440488.
- ↑ "Effects of arecoline on testosterone release in rats". American Journal of Physiology. Endocrinology and Metabolism 295 (2): E497–E504. August 2008. doi:10.1152/ajpendo.00045.2008. PMID 18559981.
- ↑ "A protective role of arecoline hydrobromide in experimentally induced male diabetic rats". BioMed Research International 2015. 2015. doi:10.1155/2015/136738. PMID 25695047.
- ↑ "Oral and systemic health effects of compulsive areca nut use.". Neuropathology of Drug Addictions and Substance Misuse; Volume 3: General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions. Academic Press. January 2016. pp. 785–793. doi:10.1016/B978-0-12-800634-4.00078-0. ISBN 978-0-12-800634-4. "Animal models demonstrate that the primary sites for metabolism of arecoline are the liver (Giri et al., 2006; Nery, 1971) and kidneys (IARC, 2004)."
- ↑ 20.0 20.1 "A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse". Chemical Research in Toxicology 19 (6): 818–827. June 2006. doi:10.1021/tx0600402. PMID 16780361.
- ↑ "Betel nut constituents as inhibitors of gamma-aminobutyric acid uptake". Nature 258 (5536): 627–628. December 1975. doi:10.1038/258627a0. PMID 1207742. Bibcode: 1975Natur.258..627J.
- ↑ "Oral and Systemic Health Effects of Compulsive Areca Nut Use". Neuropathology of Drug Addictions and Substance Misuse. 2016. pp. 785–793. doi:10.1016/B978-0-12-800634-4.00078-0. ISBN 978-0-12-800634-4.
- ↑ "Development and validation of a rapid LC-MS/MS method for simultaneous quantification of arecoline and its two active metabolites in rat plasma and its application to a pharmacokinetic study". Journal of Pharmaceutical and Biomedical Analysis 154: 397–403. May 2018. doi:10.1016/j.jpba.2018.03.033. PMID 29573735.
- ↑ Howland KL, US patent 2506458, issued 2 May 1950, assigned to Nopco Chemical Co.
- ↑ "Improvement of the synthesis of arecoline from nicotinic acid.". Pharmaceutical Chemistry Journal 10 (11): 1515–1516. November 1976. doi:10.1007/BF00760390.
- ↑ Liu N, Li J, Liu C, CN patent 105439941, published 30 March 2016, assigned to QINGDAO KANGYUAN PHARMACEUTICAL CO Ltd.
- ↑ "An improved method of preparation of arecoline, starting from acetaldehyde (exchange of experience).". Pharmaceutical Chemistry Journal 13 (11): 1158–1159. November 1979. doi:10.1007/BF00778093.
- ↑ Ward; Neal, Process for making paroxetine, U.S. Patent 6,172,233, 2001.
- ↑ Ward Neal, process of the preparation of 3-substituted-4-aryl piperidine compounds, WO 0232870, 2002.
- ↑ Coffen, David L.; Hengartner, Urs; Katonak, David A.; Mulligan, Mary E.; Burdick, David C.; Olson, Gary L.; Todaro, Louis J. (1984). "Syntheses of an antipsychotic pyrrolo[2,3-g]isoquinoline from areca alkaloids". The Journal of Organic Chemistry 49 (26): 5109–5113. doi:10.1021/jo00200a019.
- ↑ Peter Moldt, Frank Watjen, & Jorgen Scheel-Kruger, WO1998051668 (to NTG Nordic Transport Group AS).
- ↑ Frank Wätjen, et al. WO2004039778 (to NTG Nordic Transport Group AS).
- ↑ 33.0 33.1 "Muscarinic Acetylcholine Receptor Agonists as Novel Treatments for Schizophrenia". The American Journal of Psychiatry 179 (9): 611–627. September 2022. doi:10.1176/appi.ajp.21101083. PMID 35758639.
- ↑ 34.0 34.1 "Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia". The British Journal of Psychiatry 138 (1): 46–50. January 1981. doi:10.1192/bjp.138.1.46. PMID 7023592.
- ↑ "中华人民共和国农业部公告 第1845号". 2012-11-20. http://www.moa.gov.cn/nybgb/2012/dsyq/201805/t20180516_6142383.htm.
 |
