Chemistry:Myosmine
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
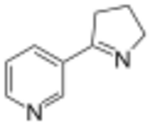
3-(3,4-Dihydro-2H-pyrrol-5-yl)pyridine | |
| Other names
3-(1-Pyrrolin-2-yl)pyridine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10N2 | |
| Molar mass | 146.193 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Isomyosamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Myosmine is an alkaloid found in tobacco[2] and other plants.[3] Chemically, it is closely related to nicotine. It inhibits aromatase sevenfold more potently than nicotine.[4] It also releases dopamine in adult but not adolescent rats.[5]
See also
References
- ↑ "Myosmine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/442649#section=Safety-and-Hazards.
- ↑ C. Laszlo, K. Kaminski, H. Guan, M. Fatarova, J. Wei, A. Bergounioux, W. K. Schlage, S. Schorderet-Weber, P. A. Guy, N. V. Ivanov, K. Lamottke and J. Hoeng (Nov 2022). "Fractionation and Extraction Optimization of Potentially Valuable Compounds and Their Profiling in Six Varieties of Two Nicotiana Species", Molecules 2022, 27(22), 8105. [1]
- ↑ Tyroller, Stefan; Zwickenpflug, Wolfgang; Richter, Elmar (2002). "New Sources of Dietary Myosmine Uptake from Cereals, Fruits, Vegetables, and Milk". Journal of Agricultural and Food Chemistry 50 (17): 4909–15. doi:10.1021/jf020281p. PMID 12166981.
- ↑ "Inhibition of human aromatase by myosmine". Drug Metabolism Letters 3 (2): 83–6. April 2009. doi:10.2174/187231209788654045. PMID 19601869.
- ↑ "Tobacco's minor alkaloids: Effects on place conditioning and nucleus accumbens dopamine release in adult and adolescent rats". European Journal of Pharmacology 814: 196–206. November 2017. doi:10.1016/j.ejphar.2017.08.029. PMID 28844873.
 |
