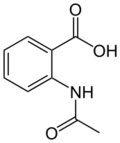
Chemistry:N-Acetylanthranilic acid
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Acetamidobenzoic acid | |||
| Other names
2-Acetamidobenzoic acid; 2-Carboxyacetanilide; o-Acetoaminobenozic acid; Acetylanthranilic acid; 2-(Acetylamino)benzoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C9H9NO3 | |||
| Molar mass | 179.175 g·mol−1 | ||
| Appearance | Slightly beige solid | ||
| Density | 1.36 g/mL | ||
| Melting point | 184 to 186 °C (363 to 367 °F; 457 to 459 K) | ||
| Boiling point | 399 °C (750 °F; 672 K) | ||
| Hazards | |||
| Safety data sheet | - | ||
| GHS pictograms | 
| ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
Oral, mouse = 1114 mg/kg | ||
| Legal status | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
N-Acetylanthranilic acid is an organic compound with the molecular formula C9H9NO3. It is an intermediate product in catabolism of quinaldine in Arthrobacter sp., and is further metabolized to anthranilic acid.[1][2]
N-Acetylanthranilic acid can be synthesized from 2-bromoacetanilide via palladium-catalyzed carbonylation in tri-n-butylamine-water at 110–130 °C, under 3 atm of carbon monoxide.[3] In the laboratory, it can be easily synthesized from anthranilic acid and acetic anhydride.
N-Acetylanthranilic acid exhibits triboluminescence when crushed.[4] The fractured crystals have large electrical potentials between areas of high and low charge. When the electrons suddenly migrate to neutralize these potentials, flashes of deep blue light are created.
In the United States, it is a Drug Enforcement Administration-controlled List I chemical,[5] because it has been used in the synthesis of methaqualone.
See also
References
- ↑ "Microbial metabolism of quinoline and related compounds. VI. Degradation of quinaldine by Arthrobacter sp". Biol Chem Hoppe-Seyler 371 (10): 1005–1008. 1990. doi:10.1515/bchm3.1990.371.2.1005. PMID 2076195.
- ↑ "Identification of large linear plasmids in Arthrobacter spp. encoding the degradation of quinaldine to anthranilate". Microbiology 151 (2): 491–500. 2005. doi:10.1099/mic.0.27521-0. PMID 15699198.
- ↑ Donald Valentine; Jefferson W. Tilley; Ronald A. LeMahieu (1981). "Practical, catalytic synthesis of anthranilic acids". Journal of Organic Chemistry 46 (22): 4614–4617. doi:10.1021/jo00335a075.
- ↑ "N-acetylanthranilic acid. A highly triboluminescent material". J Chem Educ 49 (10): 688. Oct 1972. doi:10.1021/ed049p688.
- ↑ "PART 1310 - Section 1310.02 Substances covered". http://www.deadiversion.usdoj.gov/21cfr/cfr/1310/1310_02.htm.
 |