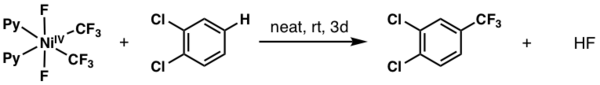Chemistry:Ni(IV) organometallic complex
Ni(IV) organometallic complexes (Nickel(IV)-centered organometallic complexes) are a form of organonickel compounds which feature a central nickel atom at the +4 oxidation state. These complexes typically need to be supported by non-canonical ligand scaffolds which are usually developed fit-for-purpose. These types of complexes are rare, with only few reports in the chemical literature when compared to lower oxidation state Ni species.[1] These types of high-valent nickel compounds have risen in popularity only in the past decade due to their general instability, challenging characterization, and unique reactivity with organic molecules. Organic reactions exploiting the reactivity of nickel typically occur via organometallic Ni0, NiI, NiII, and NiIII intermediate species. These types of species are well studied and have been the subject of several literature reviews, with few regarding NiIV species.[1][2]
Representative preparation
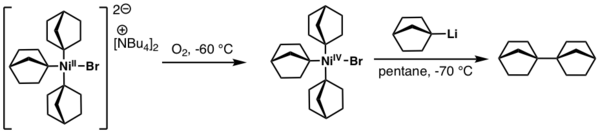
Typically, NiIV complexes are prepared from a much more accessible precursory NiII complex via a two electron oxidation process. The nature of the oxidant required relies heavily upon the ligands surrounding the Ni center, but can range from air to more exotic oxidants, such as S-(trifluoromethyl)dibenzothiophenium triflate, also known as the Umemoto reagent. For example, one of the first isolated NiIV complexes that was used in a cross coupling transformation was reported by Linden and Dimitrov in 2003[3] and was prepared by simple air-induced oxidation of the following anionic NiII complex:
Reactivity and synthetic transformations
NiIV, although elusive, features an interesting and unique reactivity profile compared to its lower valent Ni counterparts. While the cross-coupling reactivity of lower valent nickel species have been thoroughly investigated and have found vast historical and contemporary applications,[1] NiIV provides an orthogonal mode of reactivity, enabling carbon-heteroatom bonds to be formed, which would be difficult through a lower-valent Ni intermediate.[4] Only recently has the synthetic potential of NiIV been explored. There are currently no examples in total synthesis that use NiIV to enable a particular transformation.
Carbon-heteroatom bond formation
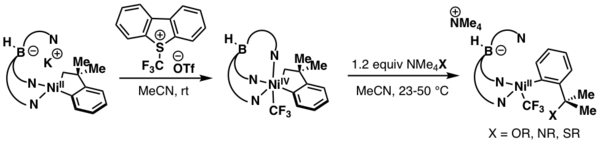
Studies reported by Sanford and Camasso in 2015 revealed the ability of a NiIV organometallic complex to deliver carbon(sp3)-oxygen, carbon(sp3)-nitrogen, and carbon(sp3)-sulfur bonds. The NiIV species was formed using Umemoto reagent as an oxidant and a trispyrazolylborate ligand.[4]
C-H activation
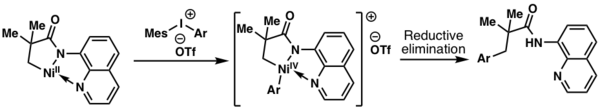
In 2014, Chatani and coworkers demonstrated that a NiIV intermediate was formed during an aminoquinoline-directed aliphatic C-H activation process using diaryliodonium salts as an oxidant.[5]
Trifluoromethylations
A handful of examples exist in the literature demonstrating how NiIV can perform challenging trifluoromethylation reactions on (hetero)arenes. A representative method is shown below, reported by Nebra and coworkers in 2017. Here, a six-coordinate NiIV complex undergoes stoichiometric CF3 transfer to a functionalized arene.[6]
A catalytic variant was reported by Sanford and coworkers in 2019. The mechanism for this transformation is shown in the following scheme. Here, the key NiIV intermediate undergoes a slow homolysis to produce a NiIII intermediate and a trifluoromethyl radical, which can promote propagation of the catalytic cycle.[7]
References
- ↑ 1.0 1.1 1.2 Nebra, Noel (2020-03-04). "High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art". Molecules 25 (5): 1141. doi:10.3390/molecules25051141. ISSN 1420-3049. PMID 32143336. PMC 7179250. http://dx.doi.org/10.3390/molecules25051141.
- ↑ Diccianni, Justin B.; Diao, Tianning (December 2019). "Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions". Trends in Chemistry 1 (9): 830–844. doi:10.1016/j.trechm.2019.08.004. ISSN 2589-5974. http://dx.doi.org/10.1016/j.trechm.2019.08.004.
- ↑ Dimitrov, Vladimir; Linden, Anthony (2003-06-16). "A Pseudotetrahedral, High-Oxidation-State Organonickel Compound: Synthesis and Structure of Bromotris(1-norbornyl)nickel(IV)". Angewandte Chemie International Edition 42 (23): 2631–2633. doi:10.1002/anie.200219383. ISSN 1433-7851. PMID 12813738. http://dx.doi.org/10.1002/anie.200219383.
- ↑ 4.0 4.1 Camasso, N. M.; Sanford, M. S. (2015-02-05). "Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes". Science 347 (6227): 1218–1220. doi:10.1126/science.aaa4526. ISSN 0036-8075. PMID 25766226. Bibcode: 2015Sci...347.1218C. http://dx.doi.org/10.1126/science.aaa4526.
- ↑ Iyanaga, Miki; Aihara, Yoshinori; Chatani, Naoto (May 2015). "ChemInform Abstract: Direct Arylation of C(sp3)-H Bonds in Aliphatic Amides with Diaryliodonium Salts in the Presence of a Nickel Catalyst.". ChemInform 46 (21): no. doi:10.1002/chin.201521038. ISSN 0931-7597. http://dx.doi.org/10.1002/chin.201521038.
- ↑ D'Accriscio, Florian; Borja, Pilar; Saffon-Merceron, Nathalie; Fustier-Boutignon, Marie; Mézailles, Nicolas; Nebra, Noel (2017-09-06). "C−H Bond Trifluoromethylation of Arenes Enabled by a Robust, High-Valent Nickel(IV) Complex". Angewandte Chemie International Edition 56 (42): 12898–12902. doi:10.1002/anie.201706237. ISSN 1433-7851. PMID 28815889. http://dx.doi.org/10.1002/anie.201706237.
- ↑ Meucci, Elizabeth A.; Nguyen, Shay N.; Camasso, Nicole M.; Chong, Eugene; Ariafard, Alireza; Canty, Allan J.; Sanford, Melanie S. (2019-08-05). "Nickel(IV)-Catalyzed C–H Trifluoromethylation of (Hetero)arenes". Journal of the American Chemical Society 141 (32): 12872–12879. doi:10.1021/jacs.9b06383. ISSN 0002-7863. PMID 31379153.