Chemistry:Nickel(II) thiocyanate
 Sample of nickel(II) thiocyanate
| |
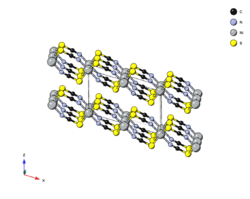 Crystal structure of nickel(II) thiocyanate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Ni(SCN)2 | |
| Molar mass | 174.86 g/mol[1] |
| Appearance | green-brown powder |
| Density | 2.59 g/cm3[1] |
| Melting point | decomposes[1] |
| 5×10−3 cm3/mol[2] | |
| Structure | |
| Hg(SCN)2 structure | |
| Octahedral | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H317, H334, H341, H350i, H360D, H372, H410 | |
| P201, P202, P260, P261, P264, P270, P272, P273, P280, P281, P285, P302+352, P304+341, P308+313, P314, P321, P333+313, P342+311, P363, P391, P405, P501 | |
| Related compounds | |
Other anions
|
Nickel(II) bromide, Nickel(II) chloride, Nickel(II) iodide |
Other cations
|
Copper(I) thiocyanate, Cobalt(II) thiocyanate, Mercury(II) thiocyanate, Ammonium thiocyanate Potassium thiocyanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nickel(II) thiocyanate is a coordination polymer with formula Ni(SCN)2.[1] It is a green-brown solid and its crystal structure was determined first in 1982.[1]
Structure
The structure of Ni(SCN)2 was determined via single-crystal X-ray diffraction and consists of two-dimensional sheets held together through Van der Waals forces. It belongs to mercury thiocyanate structure-type and can be considered a distorted form of the NiBr2 (CdI2) structure. Each nickel is octahedrally coordinated by four sulfurs and two nitrogens. The sulfur end of the SCN− ligand is doubly bridging.[1]
Synthesis
Nickel(II) thiocyanate can be prepared via salt metathesis using the reaction of methanolic solutions of KSCN and nickel(II) perchlorate hexahydrate, filtering off the precipitated KClO4 to yield a solution of Ni(SCN)2. On removal of the methanol, a pure microcrystalline powder of Ni(SCN)2 can be obtained.
Magnetism
Nickel(II) thiocyanate, like nickel(II) iodide, nickel(II) bromide and nickel(II) chloride, is an antiferromagnet at low temperatures.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Dubler, Erich; Relier, Armin; Oswald, H. R. (1982-01-01). "Intermediates in thermal decomposition of nickel(II) complexes: The crystal structures of Ni(SCN)2(NH3)2 and Ni(SCN)2". Zeitschrift für Kristallographie – Crystalline Materials 161 (1–4): 265–278. doi:10.1524/zkri.1982.161.14.265. ISSN 2196-7105.
- ↑ 2.0 2.1 DeFotis, G. C.; Dell, K. D.; Krovich, D. J.; Brubaker, W. W. (1993-05-15). "Antiferromagnetism of Ni(SCN)2". Journal of Applied Physics 73 (10): 5386–5388. doi:10.1063/1.353740. ISSN 0021-8979. Bibcode: 1993JAP....73.5386D.
 |

