Chemistry:Ammonium tetrafluoroborate
From HandWiki
Short description: Chemical compound
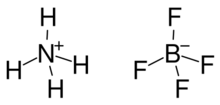
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonium tetrafluoroborate
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1759 3077 | ||
| |||
| |||
| Properties | |||
| NH4BF4 | |||
| Molar mass | 104.85 g/mol | ||
| Appearance | Colorless to white crystals[1] | ||
| Density | 1.871 g/cm3 | ||
| Melting point | 220-230 °C (Sublimes)[2] | ||
| Boiling point | N/A | ||
| 3.09 g/100 ml (-1.0 °C) 5.26 g/100 ml (-1.5 °C) 10.85 g/100 ml (-2.7 °C) 12.20 g/100 ml (0 °C) 25 g/100 ml (16 °C) 25.83 g/100 ml (25 °C) 44.09 g/100 ml (50 °C) 67.50 g/100 ml (75 °C) 98.93 g/100 ml (100 °C) 113.7 g/100 ml (108.5 °C) | |||
| Solubility | Ammonium hydroxide[3] | ||
| Hazards | |||
| Main hazards | Corrosive, irritant, toxic if ingested | ||
| Safety data sheet | [1] | ||
| GHS pictograms |  
| ||
| GHS Signal word | Warning | ||
| H290, H314, H315, H319, H335 | |||
| P234, P260, P261, P264, P271, P280, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P332+313, P337+313, P362, P363, P390, P403+233, P404, P405, P501 | |||
| Related compounds | |||
Other anions
|
Tetrafluoroborate | ||
Other cations
|
Ammonium | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ammonium tetrafluoroborate (or ammonium fluoroborate) is an inorganic salt composed of the ammonium cation and the tetrafluoroborate anion, with the chemical formula NH4BF4. When heated to decomposition, ammonium tetrafluoroborate releases toxic fumes of hydrogen fluoride, nitrogen oxides, and ammonia.[4]
Preparation
Ammonium tetrafluoroborate can be prepared by reacting ammonium fluoride with boric and sulfuric acid:[5]
- 8 NH4F + 2 H3BO3 + 3 H2SO4 → 2 NH4BF4 + 3 (NH4)2SO4 + 6 H2O
References
- ↑ "Ammonium Fluoroborate". https://www.americanelements.com/ammonium-fluoroborate-13826-83-0.
- ↑ Gregory, K. Friestad; Branchaud, Bruce P. (15 April 2001). "Ammonium Tetrafluoroborate". Encyclopedia of Reagents for Organic Synthesis.
- ↑ Lewis, R. J. (1999). Sax's Dangerous Properties of Industrial Materials. 1-3 (10 ed.). New York, NY: Van Nostrand Reinhold. pp. 233.
- ↑ Lewis, R. J. (1997). Sax's Dangerous Properties of Industrial Materials. 1-3 (9 ed.). New York, NY: Van Nostrand Reinhold. pp. 209.
- ↑ "Preparation of ammonium fluoroborate". https://prepchem.com/synthesis-of-ammonium-fluoroborate/.
 |



