Chemistry:Tropylium tetrafluoroborate
From HandWiki

| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cycloheptatrienylium tetrafluoroboranuide | |||
| Other names
Cycloheptatrienyl tetrafluoroborate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| [C 7H 7]+ [BF 4]− | |||
| Molar mass | 177.94 g·mol−1 | ||
| Appearance | white solid | ||
| Melting point | 200 °C (392 °F; 473 K) decomposition | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Danger | ||
| H314 | |||
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |||
| Related compounds | |||
Other anions
|
Tetrafluoroborate | ||
Other cations
|
Tropylium | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
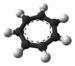
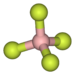
Tropylium tetrafluoroborate is an organic compound with the formula [C
7H
7]+
[BF
4]−
. Containing the tropylium cation and the non-coordinating tetrafluoroborate counteranion, tropylium tetrafluoroborate is a rare example of a readily isolable carbocation. It is a white solid.[1]
This compound may be prepared by the reaction of cycloheptatriene with phosphorus pentachloride, followed by tetrafluoroboric acid.[1][2]
See also
- Triphenylmethyl chloride, also known as trityl chloride.
References
- ↑ Jump up to: 1.0 1.1 Kenneth Conrow (1963). "Tropylium Tetrafluoroborate". Org. Synth. 43: 101. doi:10.15227/orgsyn.043.0101.
- ↑ Kane, John L.; Danheiser, Rick L. (2001). "Encyclopedia of Reagents for Organic Synthesis". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt429. ISBN 0-471-93623-5.
 |