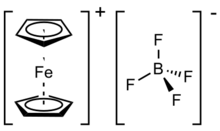
Chemistry:Ferrocenium tetrafluoroborate
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Ferrocenium tetrafluoroborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| Properties | |
| C10H10BFeF4 | |
| Molar mass | 272.84 g/mol |
| Appearance | dark blue powder |
| Melting point | 178 °C (352 °F; 451 K) (decomposes) |
| Solubility in acetonitrile | Soluble[citation needed] |
| Hazards[1] | |
| Safety data sheet | External MSDS |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P280, P305+351+338, P310 | |
| Related compounds | |
Related compounds
|
Ferrocene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ferrocenium tetrafluoroborate is an organometallic compound with the formula [Fe(C5H5)2]BF4. This salt is composed of the cation [Fe(C5H5)2]+ and the tetrafluoroborate anion (BF−4). The related hexafluorophosphate is also a popular reagent with similar properties. The ferrocenium cation is often abbreviated Fc+ or Cp2Fe+. The salt is deep blue in color and paramagnetic. Ferrocenium salts are sometimes used as one-electron oxidizing agents, and the reduced product, ferrocene, is inert and readily separated from ionic products. The ferrocene–ferrocenium couple is often used as a reference in electrochemistry. The standard potential of ferrocene-ferrocenium is dependent on specific electrochemical conditions.[2]
Preparation
Commercially available, this compound may be prepared by oxidizing ferrocene typically with ferric salts followed by addition of fluoroboric acid.[2] A variety of other oxidants work well also, such as nitrosyl tetrafluoroborate.[3] Many analogous ferrocenium salts are known.[4]
Structure
According to X-ray crystallography, the structures of the metallocene component of FcBF4 and the parent ferrocene are very similar. The Fe-C distances in the cation are 209.5 pm, about 2% longer than the Fe-C distances in ferrocene. [5]
References
- ↑ "Ferrocenium tetrafluoroborate 482358". https://www.sigmaaldrich.com/catalog/product/aldrich/482358.
- ↑ 2.0 2.1 Connelly, N. G.; Geiger, W. E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chemical Reviews 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ↑ Nielson, Roger M.; McManis, George E.; Safford, Lance K.; Weaver, Michael J. (1989). "Solvent and electrolyte effects on the kinetics of ferrocenium-ferrocene self-exchange. A reevaluation". J. Phys. Chem. 93 (5): 2152. doi:10.1021/j100342a086.
- ↑ Le Bras, J.; Jiao, H.; Meyer, W. E.; Hampel, F.; Gladysz, J. A. (2000). "Synthesis, Crystal Structure, and Reactions of the 17-Valence-Electron Rhenium Methyl Complex [(η5-C5Me5)Re(NO)(P(4-C6H4CH3)3)(CH3)]+ B(3,5-C6H3(CF3)2)−4: Experimental and Computational Bonding Comparisons with 18-Electron Methyl and Methylidene Complexes". J. Organomet. Chem. 616: 54–66. doi:10.1016/S0022-328X(00)00531-3.
- ↑ Scholz, Stefan; Scheibitz, Matthias; Schödel, Frauke; Bolte, Michael; Wagner, Matthias; Lerner, Hans-Wolfram (2007). "Difference in Reactivity of Triel Halides EX3 Towards Ferrocene". Inorganica Chimica Acta 360 (10): 3323–3329. doi:10.1016/j.ica.2007.03.049.
 |
