Chemistry:Octafluoropropane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Octafluoropropane | |||
| Other names
Freon 218
Perfluoropropane RC 218, PFC 218 R-218 Flutec PP30 genetron 218 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C3F8 | |||
| Molar mass | 188.020 g·mol−1 | ||
| Appearance | Colorless gas with faintly sweet odor | ||
| Density | 8.17 g/L | ||
| Melting point | −147.6 °C (−233.7 °F; 125.5 K) | ||
| Boiling point | −36.7 °C (−34.1 °F; 236.5 K) | ||
| Critical point (T, P) | 345.05 K (71.90 °C), 26.8 bar | ||
| Vapor pressure | 792 kPa (21.1 °C)[1] | ||
| Thermal conductivity | 13.8 mW/(m·K)[1] | ||
| Viscosity | 0.000125 Poise (0 °C)[1] | ||
| Structure | |||
| 0.014 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
149 J/(mol·K) | ||
| Hazards | |||
| Main hazards | Suffocation | ||
| GHS pictograms | 
| ||
| H280 | |||
| P410+403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | N/A | ||
| Related compounds | |||
Related halocarbons
|
Tetrafluoromethane Hexafluoroethane | ||
Related compounds
|
Propane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Octafluoropropane (C3F8) is the perfluorocarbon counterpart to the hydrocarbon propane. This non-flammable synthetic material has applications in semiconductor production and medicine. It is also an extremely potent greenhouse gas.
Manufacture
Octafluoropropane can be produced either by electrochemical fluorination or by the Fowler process using cobalt fluoride.[2]
Applications
In the electronics industry, octafluoropropane is mixed with oxygen and used as a plasma etching material for SiO2 layers in semiconductor applications, as oxides are selectively etched versus their metal substrates.[3]
In medicine, octafluoropropane may compose the gas cores of microbubble contrast agents used in contrast-enhanced ultrasound. Octafluoropropane microbubbles reflect sound waves well and are used to improve the ultrasound signal backscatter.
It is used in eye surgery, such as pars plana vitrectomy procedures where a retina hole or tear is repaired. The gas provides a long-term tamponade, or plug, of a retinal hole or tear and allows re-attachment of the retina to occur over the several days following the procedure.
Under the name R-218, octafluoropropane is used in other industries as a component of refrigeration mixtures.
It has been featured in some plans for terraforming Mars. With a greenhouse gas effect 24,000 times greater than carbon dioxide (CO2), octafluoropropane could dramatically reduce the time and resources it takes to terraform Mars.[4]
It is the active liquid in PICO-2L dark matter bubble detector (joined PICASSO and COUPP collaborations).
Major hazards
Gallery
-
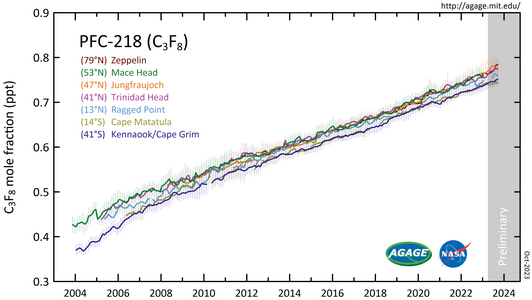
PFC-218 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion.
References
- ↑ 1.0 1.1 1.2 "Encyclopédie des gaz". air liquide. http://encyclopedia.airliquide.com/encyclopedia.asp?GasID=47&CountryID=19&LanguageID=2.
- ↑ "Synthesis of Fluorocarbons". Ind. Eng. Chem. 39 (3): 292–298. 1947. doi:10.1021/ie50447a612.
- ↑ Coburn, J. W. (1982). "Plasma-assisted etching". Plasma Chemistry and Plasma Processing 2 (1): 1–41. doi:10.1007/BF00566856.
- ↑ D. Rogers (17–21 October 2005). "Studies in the Future of Experimental Terraforming". 56th International Astronautical Congress of the International Astronautical Federation. Fukuoka, Japan: International Academy of Astronautics, and the International Institute of Space Law. http://pdf.aiaa.org/preview/CDReadyMIAF05_1429/PVIAC-05-D4.1.06.pdf.[yes|permanent dead link|dead link}}]
External links
 |