Chemistry:Odanacatib
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
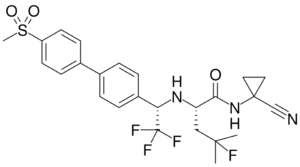
| Other names | (2S)-N-(1-Cyanocyclopropyl)-4-fluoro-4-methyl-2-{[(1S)-2,2,2-trifluoro-1-{4'-(methanesulfonyl)-[1,1'-biphenyl]-4-yl}ethyl]amino}pentanamide |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H27F4N3O3S |
| Molar mass | 525.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Odanacatib (INN;[1] codenamed MK-0822) is an investigational treatment for osteoporosis and bone metastasis.[2] It is an inhibitor of cathepsin K,[3] an enzyme involved in bone resorption.
The drug was developed by Merck & Co. The phase III clinical trial for this medicine was stopped early after a review showed it was highly effective and had a good safety profile. Merck announced in 2014 that it would apply for regulatory approval in 2015.[4]
In 2016, Merck discontinued development of odanacatib and announced it would not seek regulatory approval after analysis discovered an increased risk of stroke.[5]
This drug was developed at Merck Frosst in Montreal .
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names: List 60". WHO Drug Information (World Health Organization) 33 (3): 239. 2008. https://www.who.int/medicines/publications/druginformation/innlists/RL60.pdf. Retrieved 11 November 2016.
- ↑ "Cathepsin K inhibitors as treatment of bone metastasis". Current Opinion in Supportive and Palliative Care 2 (3): 218–222. September 2008. doi:10.1097/SPC.0b013e32830baea9. PMID 18685424.
- ↑ "The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K". Bioorganic & Medicinal Chemistry Letters 18 (3): 923–928. February 2008. doi:10.1016/j.bmcl.2007.12.047. PMID 18226527.
- ↑ "Merck osteoporosis drug passes trial, but side effects hover". 15 September 2014. https://www.reuters.com/article/us-merck-osteoporosis-idUSKBN0HA1Y820140915.
- ↑ "Merck Provides Update on Odanacatib Development Program". Business Wire. 2016-09-02. http://www.businesswire.com/news/home/20160902005107/en/Merck-Update-Odanacatib-Development-Program.
 |
