Chemistry:Oligomycin
| Error creating thumbnail: Unable to save thumbnail to destination | |
| Names | |
|---|---|
| IUPAC name
(1R,4E,5'S,6S,6'S,7R,8S,10R,11R,12S,14R,15S,16R,18E,20E,22R,25S,27R,28S,29R)-22-ethyl-7,11,14,15-tetrahydroxy-6'-[(2R)-2-hydroxypropyl]-5',6,8,10,12,14,16,28,29-nonamethyl-3',4',5',6'-tetrahydro-3H,9H,13H-spiro[2,26-dioxabicyclo[23.3.1]nonacosa-4,18,20-triene-27,2'-pyran]-3,9,13-trione
| |
| Other names
Oligomycin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | Oligomycins |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C45H74O11 | |
| Molar mass | 791.062 g/mol |
| Hazards | |
| Safety data sheet | MSDS at Fermentek |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oligomycins are macrolides created by Streptomyces that are strong antibacterial agents but are often poisonous to other organisms, including humans.
Function
Oligomycins have use as antibiotics. However, in humans, they have limited or no clinical use due to their toxic effects on mitochondria and ATP synthase.[1]
Oligomycin A is an inhibitor of ATP synthase.[1] In oxidative phosphorylation research, it is used to prevent state 3 (phosphorylating) respiration. Oligomycin A inhibits ATP synthase by blocking its proton channel (FO subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production). The inhibition of ATP synthesis by oligomycin A will significantly reduce electron flow through the electron transport chain; however, electron flow is not stopped completely due to a process known as proton leak or mitochondrial uncoupling.[2] This process is due to facilitated diffusion of protons into the mitochondrial matrix through an uncoupling protein such as thermogenin, or UCP1.
Administering oligomycin to rats can result in very high levels of lactate accumulating in the blood and urine.[3]
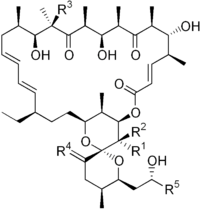
| |||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |
| Oligomycin A | CH3 | H | OH | H,H | CH3 |
| Oligomycin B | CH3 | H | OH | O | CH3 |
| Oligomycin C | CH3 | H | H | H,H | CH3 |
| Oligomycin D (Rutamycin A) |
H | H | OH | H,H | CH3 |
| Oligomycin E | CH3 | OH | OH | O | CH3 |
| Oligomycin F | CH3 | H | OH | H,H | CH2CH3 |
| Rutamycin B | H | H | H | H,H | CH3 |
| 44-Homooligomycin A | CH2CH3 | H | OH | H,H | CH3 |
| 44-Homooligomycin B | CH2CH3 | H | OH | O | CH3 |
References
- ↑ 1.0 1.1 "Inhibitors of ATP Synthase as New Antibacterial Candidates". Antibiotics 12 (4): 650. March 2023. doi:10.3390/antibiotics12040650. PMID 37107012.
- ↑ "Mitochondrial proton and electron leaks". Essays in Biochemistry 47 (1): 53–67. 2010. doi:10.1042/bse0470053. PMID 20533900.
- ↑ "Oligomycin toxicity in intact rats". Agents and Actions 15 (5–6): 660–663. December 1984. doi:10.1007/BF01966788. PMID 6532186.
- ↑ "Synthetic studies on oligomycins. Synthesis of the oligomycin B spiroketal and polypropionate portions". Bulletin of the Chemical Society of Japan 68 (3): 967–89. 1995. doi:10.1246/bcsj.68.967.
it:Fosforilazione ossidativa#Inibitori
 |
