Chemistry:Ortho-Carborane
| |
 Contaminated orthocarborane
| |
| Names | |
|---|---|
| Other names
1,2-Dicarbadodecaborane(12), ortho-dicarbadodecaborane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| |
| |
| Properties | |
| C2H12B10 | |
| Molar mass | 144.22 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 320 °C (608 °F; 593 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H228, H302, H312, H332 | |
| P210, P240, P241, P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P370+378, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
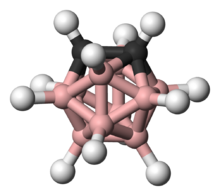
ortho-Carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C.
Structure
The cluster has C2v symmetry.[1]
Preparation
Ortho-carborane is prepared by the addition of acetylenes to decaborane(14). Modern syntheses involve two stages, the first involving generation of an adduct of decaborane:[2][3]
- B10H14 + 2 SEt2 → B10H12(SEt2)2 + H2
In the second stage, the alkyne is installed as the source of two carbon vertices:[3]
- B10H12(SEt2)2 + C2H2 → C2B10H12 + 2 SEt2 + H2
Substituted acetylenes can be employed more conveniently than acetylene gas. For example bis(acetoxymethyl)acetylene adds to the decarborane readily.
- B10H12(SEt2)2 + C2(CH2O2CCH3)2 → C2B10H10(CH2O2CCH3)2 + 2 SEt2 + H2
The organic substituents are removed by ester hydrolysis followed by oxidation:[2]
- 3 C2B10H10(CH2O2CH3)2 + 10 KOH + + 8 KMnO4 → 3 C2B10H12 + 6 CH3CO2K + 8 MnO2 + 6 K2CO3 + 8 H2O
Reactions
Thermal rearrangement
Upon heating to 420 °C, it rearranges to form the meta isomer. The para isomer is produced by heating to temperatures above 600 °C. thumb|280 px|left|o-dicarborane and its meta- and para-isomers (CH vertex indicated with black spheres).
Reduction and "reverse isomerization"
ortho-Carborane undergoes 2e- reduction when treated with a solution of lithium in ammonia. The result is the nido cluster 7,9-[C2B10H12]2-. In the dianion, the carbon vertices are not adjacent. The same cluster is produced by reduction of meta-carborane. Oxidation of the resulting 7,9-[C2B10H12]2- gives ortho-carborane.[4]
Deprotonation
Treatment with organolithium reagents gives the dilithio derivative.[5]
- C2B10H12 + 2 BuLi → Li2C2B10H10 + 2 BuH
This dilithiated compound reacts with a variety of electrophiles, e.g. chlorophosphines, chlorosilanes, and sulfur.[6]
Base-degradation to dicarbollide
Base degradation of ortho carborane gives the anionic 11-vertex derivative, precursor to dicarbollide complexes:[7]
- C2B10H12 + NaOEt + 2 EtOH → Na+C2B9H12− + H2 + B(OEt)3
- Na+C2B9H12− + NaH → Na2C2B8H11 + H2
Dicarbollides (C2B8H112-) function as ligands for transition metals and f-elements.[8] The dianion forms sandwich compounds, bis(dicarbollides). Dicarbollides, being strong electron donors, stabilize higher oxidation states, e.g. Ni(IV).
Deprotonation of carborane
The CH vertices of closo-dicarbadodecaboranes undergo deprotonation upon treatment with organolithium reagents:[9]
- C2B10H12 + 2 BuLi → Li2C2B10H10 + 2 BuH
These dilithiated compounds react with a variety of electrophiles, e.g. chlorophosphines, chlorosilanes, and sulfur.[10] Many of the same compounds can be produced by hydroboration of alkynes:
- Li2C2B10H10 + 2 RX → R2C2B10H10 + 2 LiX
- L2B10H10 + RC2R → R2C2B10H10 + 2 L (L = MeCN, etc.)
ortho-Carborane can be converted to highly reactive carborynes with the formula B10C2H10.
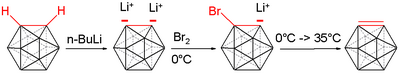
Dimerization of carborane
Upon treatment of ortho-carboranes with organolithium reagents such as n-Butyllithium, the CH vertices of the carborane cage can be deprotonated, affording the dilithiated ortho-carborane cage. Taking advantage of this more active carbon-lithium bond, the metalated carborane cages can then be treated with copper(I) chloride while in organic solvents, resulting in a copper-mediated carbon-carbon coupling reaction of the carborane cages. The copper salt is needed to avoid unwanted carbon-boron and boron-boron coupling reactions. The reaction mixture is allowed to stir at room temperature for two days, forming a copper-metalated carborane cage. Finally, the mixture is treated with 3M hydrochloric acid to quench the reaction process. The crude product is then purified via column chromatography and affords one half-equivalent of the carbon-carbon linked dimer of the original ortho-carborane in high yields. Worth noting is the effect of donating solvents on the yields of the reactions, as yields in solvents such as tetrahydrofuran and diethyl ether afford product in greatly decreased yields.[11]
History
The preparation of closo-dicarbadodecaboranes was reported independently by groups at Olin Corporation and the Reaction Motors Division of Thiokol Chemical Corporation working under the U.S. Air Force and published in 1963. These groups demonstrated the high stability in air of 1,2-closo-dodecaborane and related compounds, presented a general synthesis, described the transformation of substituents without destroying the carborane cluster, and demonstrated the ortho to meta isomerization.[12]
See also
References
- ↑ Davidson, M. G.; Hibbert, T. G.; Howard, J. A. K.; Mackinnon, A.; Wade, K. (1996). "Definitive crystal structures of ortho-, meta- and para-carboranes: supramolecular structures directed solely by C–H⋯O hydrogen bonding to hmpa (hmpa = hexamethylphosphoramide)". Chem. Commun. (19): 2285–2286. doi:10.1039/CC9960002285.
- ↑ Jump up to: 2.0 2.1 Charles R. Kutal David A. Owen Lee J. Todd (1968). closo‐1,2‐Dicarbadodecaborane(12). Inorganic Syntheses. 11. pp. 19–24. doi:10.1002/9780470132425.ch5.
- ↑ Jump up to: 3.0 3.1 M. Frederick Hawthorne; Timothy D. Andrews; Philip M. Garrett; Fred P. Olsen; Marten Reintjes; Fred N. Tebbe; Les F. Warren; Patrick A. Wegner et al. (1967). "Icosahedral Carboranes and Intermediates Leading to the Preparation of Carbametallic Boron Hydride Derivatives". Inorganic Syntheses. Inorganic Syntheses. 10. pp. 91–118. doi:10.1002/9780470132418.ch17. ISBN 978-0-470-13241-8.
- ↑ Russell N. Grimes (2016). "10. Icosahedral Carboranes: 1,7-C2B10H12 and 1,12-C2B10H12". Carboranes, 3rd Edition. Elsevier. ISBN 978-0-12-801905-4.
- ↑ Popescu, A.-R.; Musteti, A. D.; Ferrer-Ugalde, A.; Viñas, C.; Núñez, R.; Teixidor, F. (2012). "Influential Role of Ethereal Solvent on Organolithium Compounds: The Case of Carboranyllithium". Chemistry – A European Journal 18 (11): 3174–3184. doi:10.1002/chem.201102626. PMID 22334417.
- ↑ Jin, G.-X. (2004). "Advances in the Chemistry of Organometallic Complexes with 1,2-Dichalcogenolato-o-Carborane Ligands". Coord. Chem. Rev. 248 (7–8): 587–602. doi:10.1016/j.ccr.2004.01.002.
- ↑ Plešek, J.; Heřmánek, S.; Štíbr, B. (1983). "Potassium dodecahydro-7, 8-dicarba-nido -undecaborate(1-), k[7, 8-c2 b9 h12 ], intermediates, stock solution, and anhydrous salt". Potassium dodecahydro-7,8-dicarba-nido-undecaborate(1-), k[7,8-C2B9H12], intermediates, stock solution, and anhydrous salt. Inorganic Syntheses. 22. pp. 231–234. doi:10.1002/9780470132531.ch53. ISBN 978-0-470-13253-1.
- ↑ Hawthorne, M. F.; Young, D. C.; Wegner, P. A. (1965). "Carbametallic Boron Hydride Derivatives. I. Apparent Analogs of Ferrocene and Ferricinium Ion". Journal of the American Chemical Society 87 (8): 1818–1819. doi:10.1021/ja01086a053.
- ↑ Popescu, A.-R.; Musteti, A. D.; Ferrer-Ugalde, A.; Viñas, C.; Núñez, R.; Teixidor, F. (2012). "Influential Role of Ethereal Solvent on Organolithium Compounds: The Case of Carboranyllithium". Chemistry – A European Journal 18 (11): 3174–3184. doi:10.1002/chem.201102626. PMID 22334417.
- ↑ Jin, G.-X. (2004). "Advances in the Chemistry of Organometallic Complexes with 1,2-Dichalcogenolato-o-Carborane Ligands". Coord. Chem. Rev. 248 (7–8): 587–602. doi:10.1016/j.ccr.2004.01.002.
- ↑ Ren, Shikuo; Xie, Zuowei (2008-10-13). "A Facile and Practical Synthetic Route to 1,1′-Bis( o -carborane)" (in en). Organometallics 27 (19): 5167–5168. doi:10.1021/om8005323. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/om8005323.
- ↑ Heying, T. L.; Ager, J. W.; Clark, S. L.; Mangold, D. J.; Goldstein, H. L.; Hillman, M.; Polak, R. J.; Szymanski, J. W. (1963). "A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds". Inorganic Chemistry 2 (6): 1089–1092. doi:10.1021/ic50010a002.
 |