Chemistry:Peptoid
Peptoids (root from the Greek πεπτός, peptós "digested"; derived from πέσσειν, péssein "to digest" and the Greek-derived suffix -oid meaning "like, like that of, thing like a ______," ), or poly-N-substituted glycines, are a class of biochemicals known as biomimetics that replicate the behavior of biological molecules.[1] Peptidomimetics are recognizable by side chains that are appended to the nitrogen atom of the peptide backbone, rather than to the α-carbons (as they are in amino acids).
Chemical structure and synthesis
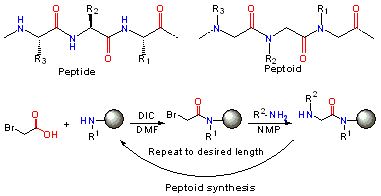
In peptoids, the side chain is connected to the nitrogen of the peptide backbone, instead of the α-carbon as in peptides. Notably, peptoids lack the amide hydrogen which is responsible for many of the secondary structure elements in peptides and proteins. Peptoids were first invented by Reyna J. Simon, Ronald N. Zuckermann, Paul Bartlett and Daniel V. Santi to mimic protein/peptide products to aid in the discovery of protease-stable small molecule drugs for the East Bay company Chiron.[2][3]
Following the sub-monomer protocol originally created by Ron Zuckermann,[4] each residue is installed in two steps: acylation and displacement. In the acylation step, a haloacetic acid, typically bromoacetic acid activated by diisopropylcarbodiimide reacts with the amine of the previous residue. In the displacement step (a classical SN2 reaction), an amine displaces the halide to form the N-substituted glycine residue. The submonomer approach allows the use of any commercially available or synthetically accessible amine with great potential for combinatorial chemistry.
Unique characteristics
Like D-Peptides and β peptides, peptoids are completely resistant to proteolysis,[5] and are therefore advantageous for therapeutic applications where proteolysis is a major issue. Since secondary structure in peptoids does not involve hydrogen bonding, it is not typically denatured by solvent, temperature, or chemical denaturants such as urea (see details below).
Notably, since the amino portion of the amino acid results from the use of any amine, thousands of commercially available amines can be used to generate unprecedented chemical diversity at each position at costs far lower than would be required for similar peptides or peptidomimetics. To date, at least 230 different amines have been used as side chains in peptoids.[6]
Structure
Peptoid oligomers are known to be conformationally unstable, due to the flexibility of the main-chain methylene groups and the absence of stabilizing hydrogen bond interactions along the backbone. Nevertheless, through the choice of appropriate side chains it is possible to form specific steric or electronic interactions that favour the formation of stable secondary structures like helices,[7] especially peptoids with C-α-branched side chains are known to adopt structure analogous to polyproline I helix.[8] Different strategies have been employed to predict and characterize peptoid secondary structure, with the ultimate goal of developing fully folded peptoid protein structures[9] The cis/trans amide bond isomerization still leads to a conformational heterogeneity which doesn’t allow for the formation of homogeneous peptoid foldamers.[10] Nonetheless, scientists were able to find trans-inducer N-Aryl side chains promoting polyproline type II helix,[11] and strong cis-inducer such as bulky naphtylethyl[12] and tert-butyl[13] side chains. It was also found that n→π* interactions can modulate the ratio of cis/trans amide bond conformers,[14] until reaching a complete control of the cis conformer in the peptoid backbone using a functionalizable triazolium side chain.[15]
Applications
The first demonstration of the use of peptoids was in screening a combinatorial library of diverse peptoids, which yielded novel high-affinity ligands for 7-transmembrane G-protein-couple receptors.[16]
Peptoids have been developed as candidates for a range of different biomedical applications,[17][18] including antimicrobial agents,[19] synthetic lung surfactants,[20][21] ligands for various proteins including Src Homology 3 (SH3 domain),[22] Vascular Endothelial Growth Factor (VEGF) receptor 2,[23] and antibody Immunoglobulin G biomarkers for the identification of Alzheimer's disease.[24]
Due to their advantageous characteristics as described above, peptoids are also being actively developed for use in nanotechnology,[25] an area in which they may play an important role.[26]
Antimicrobial agents
Researchers supported by grants from the NIH and NIAID tested the efficacy of antimicrobial peptoids against antibiotic-resistant strands of Mycobacterium tuberculosis.[27] Antimicrobial peptoids demonstrate a non-specific mechanism of action against the bacterial membrane, one that differs from small-molecule antibiotics that bind to specific receptors (and thus are susceptible to mutations or alterations in bacterial structure). Preliminary results suggested "appreciable activity" against drug-sensitive bacterial strands, leading to a call for more research into the viability of peptoids as a new class of tuberculocidal drugs.[27]
Researchers at the Barron Lab at Stanford University (supported by a NIH Pioneer Award grant) are currently studying whether upregulation of the human host defense peptide LL-37 or application of antimicrobial treatments based on LL-37 may prevent or treat sporadic Alzheimer’s dementia. Lead researcher Annelise Barron discovered that the innate human defense peptide LL-37 binds to the peptide Ab, which is associated with Alzheimer's disease. Barron's insight is that an imbalance between LL-37 and Ab may be a critical factor affecting AD-associated fibrils and plaques. The project extends focus upon the potential relationship between chronic, oral P. gingivalis and herpesvirus (HSV-1) infections to the progression of Alzheimer's dementia.
See also
References
- ↑ "What if We Could Give Viruses a One-Two Punch? by Berkeley Lab on Exposure" (in en). https://photostories.lbl.gov/what-if-we-could-give-viruses-a-onetwo-punch.
- ↑ "Peptoids: a modular approach to drug discovery". Proceedings of the National Academy of Sciences of the United States of America 89 (20): 9367–9371. October 1992. doi:10.1073/pnas.89.20.9367. PMID 1409642.
- ↑ "What if We Could Give Viruses a One-Two Punch? by Berkeley Lab on Exposure" (in en). https://photostories.lbl.gov/what-if-we-could-give-viruses-a-onetwo-punch.
- ↑ "Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis". Journal of the American Chemical Society 114 (26): 10646–10647. 1992. doi:10.1021/ja00052a076.
- ↑ "Comparison of the proteolytic susceptibilities of homologous L‐amino acid, D‐amino acid, and N‐substituted glycine peptide and peptoid oligomers.". Drug Development Research 35 (1): 20–32. May 1995. doi:10.1002/ddr.430350105.
- ↑ "Solid-phase synthesis of N-substituted glycine oligomers (alpha-peptoids) and derivatives". Molecules 15 (8): 5282–5335. August 2010. doi:10.3390/molecules15085282. PMID 20714299.
- ↑ "Sequence-specific polypeptoids: a diverse family of heteropolymers with stable secondary structure". Proceedings of the National Academy of Sciences of the United States of America 95 (8): 4303–4308. April 1998. doi:10.1073/pnas.95.8.4303. PMID 9539732.
- ↑ "NMR determination of the major solution conformation of a peptoid pentamer with chiral side chains". Proceedings of the National Academy of Sciences of the United States of America 95 (8): 4309–4314. April 1998. doi:10.1073/pnas.95.8.4309. PMID 9539733.
- ↑ "Progress in the de novo design of structured peptoid protein mimics". Biopolymers 96 (5): 556–560. 2011. doi:10.1002/bip.21621. PMID 22180903.
- ↑ "Peptoid architectures: elaboration, actuation, and application". Current Opinion in Chemical Biology 12 (6): 714–721. December 2008. doi:10.1016/j.cbpa.2008.08.015. PMID 18786652.
- ↑ "Oligo(N-aryl glycines): a new twist on structured peptoids". Journal of the American Chemical Society 130 (49): 16622–16632. December 2008. doi:10.1021/ja804580n. PMID 19049458.
- ↑ "Extraordinarily robust polyproline type I peptoid helices generated via the incorporation of α-chiral aromatic N-1-naphthylethyl side chains". Journal of the American Chemical Society 133 (39): 15559–15567. October 2011. doi:10.1021/ja204755p. PMID 21861531.
- ↑ "The tert-butyl side chain: a powerful means to lock peptoid amide bonds in the cis conformation". Organic Letters 15 (9): 2246–2249. May 2013. doi:10.1021/ol400820y. PMID 23590358.
- ↑ "New strategies for the design of folded peptoids revealed by a survey of noncovalent interactions in model systems". Journal of the American Chemical Society 131 (45): 16555–16567. November 2009. doi:10.1021/ja907184g. PMID 19860427.
- ↑ "The click triazolium peptoid side chain: a strong cis-amide inducer enabling chemical diversity". Journal of the American Chemical Society 134 (23): 9553–9556. June 2012. doi:10.1021/ja302342h. PMID 22612307.
- ↑ "Discovery of nanomolar ligands for 7-transmembrane G-protein-coupled receptors from a diverse N-(substituted)glycine peptoid library". Journal of Medicinal Chemistry 37 (17): 2678–2685. August 1994. doi:10.1021/jm00043a007. PMID 8064796.
- ↑ "Structure-function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function". Organic & Biomolecular Chemistry 7 (8): 1508–1524. April 2009. doi:10.1039/b817980h. PMID 19343235.
- ↑ "Peptoids as potential therapeutics". Current Opinion in Molecular Therapeutics 11 (3): 299–307. June 2009. PMID 19479663.
- ↑ "Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids". Pharmaceuticals 14 (4): 304. March 2021. doi:10.3390/ph14040304. PMID 33807248.
- ↑ "Biomimicry of surfactant protein C". Accounts of Chemical Research 41 (10): 1409–1417. October 2008. doi:10.1021/ar800058t. PMID 18834153.
- ↑ "Effective in vivo treatment of acute lung injury with helical, amphipathic peptoid mimics of pulmonary surfactant proteins". Scientific Reports 8 (1): 6795. May 2018. doi:10.1038/s41598-018-25009-3. PMID 29717157.
- ↑ "Exploiting the basis of proline recognition by SH3 and WW domains: design of N-substituted inhibitors". Science 282 (5396): 2088–2092. December 1998. doi:10.1126/science.282.5396.2088. PMID 9851931.
- ↑ "A peptoid "antibody surrogate" that antagonizes VEGF receptor 2 activity". Journal of the American Chemical Society 130 (17): 5744–5752. April 2008. doi:10.1021/ja711193x. PMID 18386897.
- ↑ "Identification of candidate IgG biomarkers for Alzheimer's disease via combinatorial library screening". Cell 144 (1): 132–142. January 2011. doi:10.1016/j.cell.2010.11.054. PMID 21215375.
- ↑ "Free-floating ultrathin two-dimensional crystals from sequence-specific peptoid polymers". Nature Materials 9 (5): 454–460. May 2010. doi:10.1038/nmat2742. PMID 20383129.
- ↑ "Peptoids at the 7th Summit: toward macromolecular systems engineering". Biopolymers 96 (5): 537–544. 2011. doi:10.1002/bip.21623. PMID 22180902.
- ↑ 27.0 27.1 "Efficacy of antimicrobial peptoids against Mycobacterium tuberculosis". Antimicrobial Agents and Chemotherapy 55 (6): 3058–3062. June 2011. doi:10.1128/AAC.01667-10. PMID 21464254.
 |
