Biology:SH3 domain
| SH3 domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
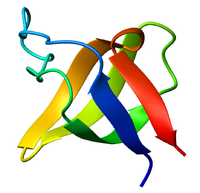 Ribbon diagram of the SH3 domain, alpha spectrin, from chicken (PDB accession code 1SHG), colored from blue (N-terminus) to red (C-terminus). | |||||||||
| Identifiers | |||||||||
| Symbol | SH3_1 | ||||||||
| Pfam | PF00018 | ||||||||
| Pfam clan | CL0010 | ||||||||
| InterPro | IPR001452 | ||||||||
| SMART | SM00326 | ||||||||
| PROSITE | PS50002 | ||||||||
| SCOP2 | 1shf / SCOPe / SUPFAM | ||||||||
| CDD | cd00174 | ||||||||
| |||||||||
The SRC Homology 3 Domain (or SH3 domain) is a small protein domain of about 60 amino acid residues. Initially, SH3 was described as a conserved sequence in the viral adaptor protein v-Crk. This domain is also present in the molecules of phospholipase and several cytoplasmic tyrosine kinases such as Abl and Src.[1][2] It has also been identified in several other protein families such as: PI3 Kinase, Ras GTPase-activating protein, CDC24 and cdc25.[3][4][5] SH3 domains are found in proteins of signaling pathways regulating the cytoskeleton, the Ras protein, and the Src kinase and many others. The SH3 proteins interact with adaptor proteins and tyrosine kinases. Interacting with tyrosine kinases, SH3 proteins usually bind far away from the active site. Approximately 300 SH3 domains are found in proteins encoded in the human genome. In addition to that, the SH3 domain was responsible for controlling protein-protein interactions in the signal transduction pathways[6] and regulating the interactions of proteins involved in the cytoplasmic signaling.[7]
Structure
The SH3 domain has a characteristic beta-barrel fold that consists of five or six β-strands arranged as two tightly packed anti-parallel β sheets. The linker regions may contain short helices. The SH3-type fold is an ancient fold found in eukaryotes as well as prokaryotes.[8]
Peptide binding
The classical SH3 domain is usually found in proteins that interact with other proteins and mediate assembly of specific protein complexes, typically via binding to proline-rich peptides in their respective binding partner. Classical SH3 domains are restricted in humans to intracellular proteins, although the small human MIA family of extracellular proteins also contain a domain with an SH3-like fold.
Many SH3-binding epitopes of proteins have a consensus sequence that can be represented as a regular expression or Short linear motif:
-X-P-p-X-P- 1 2 3 4 5
with 1 and 4 being aliphatic amino acids, 2 and 5 always and 3 sometimes being proline. The sequence binds to the hydrophobic pocket of the SH3 domain. More recently, SH3 domains that bind to a core consensus motif R-x-x-K have been described. Examples are the C-terminal SH3 domains of adaptor proteins like Grb2 and Mona (a.k.a. Gads, Grap2, Grf40, GrpL etc.). Other SH3 binding motifs have emerged and are still emerging in the course of various molecular studies, highlighting the versatility of this domain.
SH3 interactomes
SH3 domain-mediated protein-protein interaction networks, i.e., SH3 interactomes, revealed that worm SH3 interactome resembles the analogous yeast network because it is significantly enriched for proteins with roles in endocytosis.[9][10] Nevertheless, orthologous SH3 domain-mediated interactions are highly rewired between worm and yeast.[9]
Proteins with SH3 domain
- Signal transducing adaptor proteins
- CDC24
- Cdc25
- PI3 kinase
- Phospholipase
- Ras GTPase-activating protein
- Vav proto-oncogene
- GRB2
- p54 S6 kinase 2 (S6K2)
- SH3D21
- ARMH3 (potentially)
- STAC3
- Some myosins
- SH3 and multiple ankyrin repeat domains: SHANK1, SHANK2, SHANK3
- YAP1
- ARHGAP12
- vexin (VXN)
- TANGO1
- Integrase
- Focal Adhesion Kinase (FAK, PTK2)
- Proline-rich tyrosine kinase (Pyk2, CADTK, PTK2beta)
- TRIP10 (cip4)
See also
- Src homology 2 domain-containing
- Structural domain
References
- ↑ "SH2 and SH3 domains". Current Biology 3 (7): 434–42. July 1993. doi:10.1016/0960-9822(93)90350-W. PMID 15335710.
- ↑ "SH3 domains: complexity in moderation". Journal of Cell Science 114 (Pt 7): 1253–63. April 2001. doi:10.1242/jcs.114.7.1253. PMID 11256992.
- ↑ "SH3--an abundant protein domain in search of a function". FEBS Letters 307 (1): 55–61. July 1992. doi:10.1016/0014-5793(92)80901-R. PMID 1639195.
- ↑ "Signalling through SH2 and SH3 domains". Trends in Cell Biology 3 (1): 8–13. January 1993. doi:10.1016/0962-8924(93)90194-6. PMID 14731533.
- ↑ "Protein modules and signalling networks". Nature 373 (6515): 573–80. February 1995. doi:10.1038/373573a0. PMID 7531822.
- ↑ "SH2/SH3 signaling proteins". Current Opinion in Genetics & Development 4 (1): 25–30. February 1994. doi:10.1016/0959-437X(94)90087-6. PMID 8193536.
- ↑ "SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins". Science 252 (5006): 668–74. May 1991. doi:10.1126/science.1708916. PMID 1708916.
- ↑ "SH3 domains in prokaryotes". Trends in Biochemical Sciences 24 (4): 132–3. April 1999. doi:10.1016/s0968-0004(99)01366-3. PMID 10322416.
- ↑ 9.0 9.1 Xin, Xiaofeng; Gfeller, David; Cheng, Jackie; Tonikian, Raffi; Sun, Lin; Guo, Ailan; Lopez, Lianet; Pavlenco, Alevtina et al. (2013-01-01). "SH3 interactome conserves general function over specific form". Molecular Systems Biology 9: 652. doi:10.1038/msb.2013.9. ISSN 1744-4292. PMID 23549480.
- ↑ Tonikian, Raffi; Xin, Xiaofeng; Toret, Christopher P.; Gfeller, David; Landgraf, Christiane; Panni, Simona; Paoluzi, Serena; Castagnoli, Luisa et al. (2009-10-01). "Bayesian modeling of the yeast SH3 domain interactome predicts spatiotemporal dynamics of endocytosis proteins". PLOS Biology 7 (10): e1000218. doi:10.1371/journal.pbio.1000218. ISSN 1545-7885. PMID 19841731.
External links
- Eukaryotic Linear Motif resource motif class LIG_SH3_1
- Eukaryotic Linear Motif resource motif class LIG_SH3_2
- Eukaryotic Linear Motif resource motif class LIG_SH3_3
- Eukaryotic Linear Motif resource motif class LIG_SH3_4
- Eukaryotic Linear Motif resource motif class LIG_SH3_5
- Eukaryotic Linear Motif resource motif class TRG_PEX_1
- Nash Lab Protein Interaction Domains in Signal Transduction - The SH3 domain
- GENEART - Screen your protein against all human SH3 domains in a single phage display cycle
 |
