Chemistry:Perfluorobutane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Decafluorobutane[1] | |||
| Other names
Perflubutane (USAN)
DFB Halocarbon 610 R610 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4F10 | |||
| Molar mass | 238.028 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 11.21 kg/m3 (gas, 101.3 kPa at boiling point)[2] 1594 kg/m3 (liquid, 101.3 kPa at boiling point)[2] [[[Link rot |
dead link}}]]] | |
| Melting point | −128 °C (−198 °F; 145 K)[3] | ||
| Boiling point | −1.7 °C (28.9 °F; 271.4 K)[2] | ||
| 1.5 mg/L (101.3 kPa)[3] | |||
| log P | > 3.93 (n-octanol/water)[3] | ||
| Vapor pressure | 330.3 kPa (at 25 °C)[3] | ||
| Viscosity | 0.0001218 Poise[2] | ||
| Hazards | |||
| Safety data sheet | MSDS at Linde Gas | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
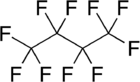
Perfluorobutane (PFB) is an inert, high-density colorless gas. It is a simple fluorocarbon with a n-butane skeleton and all the hydrogen atoms replaced with fluorine atoms.
Uses
Perfluorobutane can replace Halon 1301 in fire extinguishers,[4] as well as the gas component for newer generation microbubble ultrasound contrast agents. Sonazoid[5] is one such microbubble formulation developed by Amersham Health that uses perfluorobutane for the gas core.
Inhaling perfluorobutane makes one's voice deeper.
Environmental impacts
If perfluorobutane is released to the environment, it will not be broken down in air. It is not expected to be broken down by sunlight. It will move into air from soil and water surfaces. If it is exposed to conditions of extreme heat from misuse, equipment failure, etc., toxic decomposition products including hydrogen fluoride can be produced.[6]
Perfluorobutane has an estimated lifetime greater than 2600 years. Perfluorobutane has a high global warming potential value of 4800.[7] Its ozone depletion potential is zero.[citation needed]
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 33. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. "The prefix ‘per-’ is no longer recommended."
- ↑ 2.0 2.1 2.2 2.3 "Perfluorobutane (R610)". Gas Encyclopaedia. Air Liquide. http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=19. Retrieved November 1, 2012.
- ↑ 3.0 3.1 3.2 3.3 "Summary Report: PERFLUOROBUTANE". http://www.nicnas.gov.au/publications/car/new/na/nasummr/na0300sr/na317.asp.
- ↑ "Perfluorobutane — Full Public Report". National Industrial Chemicals Notification and Assessment Scheme. 1996. http://www.nicnas.gov.au/publications/car/new/na/nafullr/na0300fr/na317fr.pdf.pan.txt.
- ↑ "Sonoazoid - US TIP". http://www.us-tip.com/serv1.php?type=db1&dbs=sonazoid.
- ↑ "Perflubutane". https://pubchem.ncbi.nlm.nih.gov/compound/9638.
- ↑ "Global Warming Potentials (IPCC Second Assessment Report)". United Nations Framework Convention on Climate Change. https://unfccc.int/process/transparency-and-reporting/greenhouse-gas-data/greenhouse-gas-data-unfccc/global-warming-potentials.
 |