Chemistry:Perfluorocyclohexane
From HandWiki
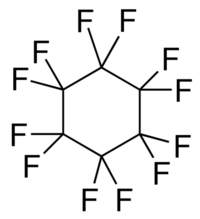
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dodecafluorocyclohexane | |
| Other names
1,1,2,2,3,3,4,4,5,5,6,6-Dodecafluorocyclohexane, Cyclohexane, dodecafluoro-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6F12 | |
| Molar mass | 300.047 g·mol−1 |
| Appearance | clear, waxy solid |
| Density | 1.684 g/cm3 |
| Melting point | 52 °C (126 °F; 325 K)[1] |
| Boiling point | 59–60 °C (138–140 °F; 332–333 K) |
| Solubility | Miscible with organic compounds |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | MSDS [1] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Fluorocarbon |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Perfluorocyclohexane or dodecafluorocyclohexane is a chemical which belongs to the class of fluorocarbons, sometimes referred to as perfluorocarbons or PFCs. Fluorocarbons and their derivatives are useful fluoropolymers, refrigerants, solvents, and anesthetics.
Synthesis
Perfluorocyclohexane can be synthesized by fluorination of cyclohexane.[2]
Properties
Perfluorocyclohexane is chemically inert and thermally stable. It is a relatively non-toxic, clear, waxy solid, which has a high vapor pressure and therefore sublimes readily at room temperature.[citation needed]
The molecule predominantly exists in its chair conformation, in which it possesses D3d molecular symmetry.
References
- ↑ Sander, M.; Blöchl, W. (January 1965). "Herstellung von Perfluoralkanen und Perfluorcycloalkanen" (in German). Chemie Ingenieur Technik 37 (1): 7–13. doi:10.1002/cite.330370103.
- ↑ Sandford G. (2003). "Perfluoroalkanes". Tetrahedron 59 (4): 437–454. doi:10.1016/s0040-4020(02)01568-5.
 |

