Chemistry:Perfluorotoluene
From HandWiki
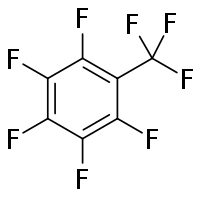
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentafluoro(trifluoromethyl)benzene | |
| Other names
Octafluorotoluene, Benzene, pentafluoro(trifluoromethyl)-, Pentafluorobenzotrifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7F8 | |
| Molar mass | 236.064 g·mol−1 |
| Appearance | Clear light yellow liquid |
| Density | 1.666 g/cm3 |
| Melting point | −65.6 °C (−86.1 °F; 207.6 K) |
| Boiling point | 104 °C (219 °F; 377 K) |
| Solubility | Miscible with Organic compounds |
| Vapor pressure | 26 mmHg |
| Hazards | |
| Main hazards | Irritant, Highly Flammable |
| Safety data sheet | MSDS [1] |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| NFPA 704 (fire diamond) | |
| Flash point | 20 °C (68 °F; 293 K) |
| Related compounds | |
Related compounds
|
Fluorocarbon |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Perfluorotoluene or octafluorotoluene is a chemical which belongs to the class of fluorocarbons, sometimes referred to as perfluorocarbons or PFCs. Fluorocarbons and their derivatives are useful fluoropolymers, refrigerants, solvents, and anesthetics.
More specifically, perfluorotoluene is a kind of perfluorocarbon, which is a type of perfluoroaromatic compounds in which they contain only carbon and fluorine like other fluorocarbons, but also contain an aromatic ring. Other examples include hexafluorobenzene and octafluoronaphthalene. Perfluorotoluene is commonly used as industrial solvent and can be prepared by defluorination of perfluoromethylcyclohexane by heating to 500 °C with a nickel or iron catalyst.[1]
References
- ↑ Banks, RE (1970). Fluorocarbons and their Derivatives, Second Edition. London: MacDonald & Co. (Publishers) Ltd.. pp. 203–207. ISBN 0-356-02798-8.
 |


