Chemistry:Phosphorus heptabromide
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
Tetrabromophosphanium tribromide
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
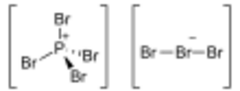
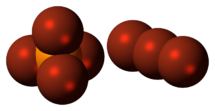
| [PBr 4]+ [Br 3]− | |
| Molar mass | 590.302 g·mol−1 |
| Appearance | Red prismatic crystals |
| Structure[1] | |
| Orthorhombic | |
| Pnma, No. 64 | |
a = 9.35 Å, b = 7.94 Å, c = 14.69 Å
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Phosphorus heptabromide is an inorganic compound with the chemical formula PBr
7. It is one of the phosphorus bromides. At normal conditions, it forms red prismatic crystals. PBr
7 can be prepared by the sublimation of a mixture of phosphorus pentabromide and bromine.[2]
- PBr
5 + Br
2 → PBr
7
The structure of PBr
7 consists of a tetrabromophosphonium cation [PBr
4]+
, paired with a tribromide anion [Br
3]−
, and the tribromide anion is non-symmetric.[1]
See also
References
- ↑ 1.0 1.1 Breneman, G. L.; Willett, R. D. (1967). "The crystal structure of phosphorus heptabromide, PBr7". Acta Crystallographica 23 (3): 467–471. doi:10.1107/S0365110X67002981.
- ↑ T. E. (Thomas Edward) Thorpe. A dictionary of applied chemistry (Volume 4)
 |

