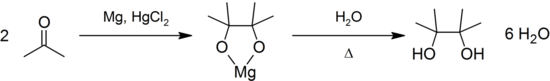Chemistry:Pinacol
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dimethylbutane-2,3-diol | |
| Other names
2,3-Dimethyl-2,3-butanediol
Tetramethylethylene glycol 1,1,2,2-Tetramethylethylene glycol Pinacone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H14O2 | |
| Molar mass | 118.174 g/mol |
| Appearance | White solid |
| Density | 0.967 g/cm3 |
| Melting point | 40 to 43 °C (104 to 109 °F; 313 to 316 K) |
| Boiling point | 171 to 173 °C (340 to 343 °F; 444 to 446 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H228, H315, H319, H335 | |
| P210, P240, P241, P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P405, P501 | |
| Flash point | 77 °C (171 °F; 350 K) |
| Related compounds | |
Related compounds
|
Pinacolone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
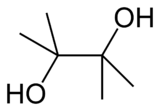
Pinacol is a white solid organic compound. It is a diol that has hydroxyl groups (-OH) on vicinal carbon atoms.
Preparation
It may be produced by the pinacol coupling reaction from acetone:[1]
Reactions
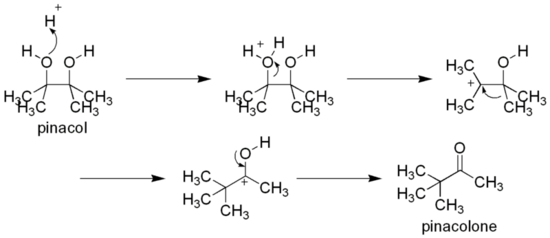
As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g. by heating with sulfuric acid:[2]
Pinacol can be used with borane and boron trichloride to produce useful synthetic intermediates such as pinacolborane, bis(pinacolato)diboron,[3] and pinacolchloroborane.
See also
References
- ↑ Roger Adams and E. W. Adams. "Pinacol Hydrate". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0459.; Collective Volume, 1, pp. 459
- ↑ G. A. Hill and E. W. Flosdorf (1941). "Pinacolone". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV1P0462.; Collective Volume, 1, pp. 462
- ↑ Tatsuo Ishiyama; Miki Murata; Taka-aki Ahiko; Norio Miyaura (2004). "Bis(pinacolato)diboron". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=v77p0176.; Collective Volume, 10, pp. 115
 |