Chemistry:Pinacol coupling reaction
| Pinacol coupling reaction | |
|---|---|
| Named after | Pinacol |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | pinacol-coupling-reaction |

A pinacol coupling reaction is an organic reaction in which a carbon–carbon bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process.[1] The reaction product is a vicinal diol. The reaction is named after pinacol (also known as 2,3-dimethyl-2,3-butanediol or tetramethylethylene glycol), which is the product of this reaction when done with acetone as reagent. The reaction is usually a homocoupling but intramolecular cross-coupling reactions are also possible. Pinacol was discovered by Wilhelm Rudolph Fittig in 1859.
Reaction mechanism
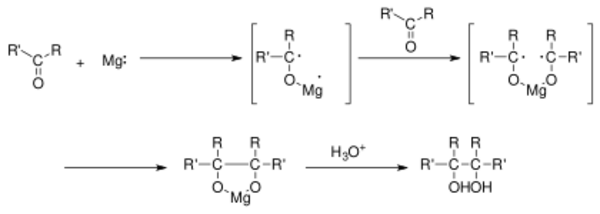
The first step in the reaction mechanism is a one-electron reduction of the carbonyl group by a reducing agent —such as magnesium— to a ketyl radical anion species. Two ketyl groups react in a coupling reaction yielding a vicinal diol with both hydroxyl groups deprotonated. Addition of water or another proton donor gives the diol. With magnesium as an electron donor, the initial reaction product is a 5-membered cyclic compound with the two oxygen atoms coordinated to the oxidized Mg2+ ion. This complex is broken up by addition of water with formation of magnesium hydroxide. The pinacol coupling can be followed up by a pinacol rearrangement. A related reaction is the McMurry reaction, which uses titanium(III) chloride or titanium(IV) chloride in conjunction with a reducing agent for the formation of the metal-diol complex, and which takes place with an additional deoxygenation reaction step in order to provide an alkene product.
Scope
Benzophenone may undergo the pinacol coupling photochemically.[2] Benzaldehyde may also be used as a substrate with the use of catalytic vanadium(III) chloride and aluminium metal as the stoichiometric reductant.[3] This heterogeneous reaction in water at room temperature yields 72% after 3 days with 56:44 dl:meso composition.
In another system with benzaldehyde, Montmorillonite K-10]] and zinc chloride in aqueous THF under ultrasound the reaction time is reduced to 3 hours (composition 55:45).[4] On the other hand, certain tartaric acid derivatives can be obtained with high diastereoselectivity in a system of samarium(II) iodide and HMPA.[5]
A titanium-catalyzed photocatalytic approach was also developed: the use of catalytic titanocene dichloride in the presence of a red-absorbing organic dye as the photosensitizer, and Hantzsch ester as the terminal reducing agent, enabled the homocoupling reactions of a wide variety of aromatic aldehydes in trifluorotoluene under orange-light irradiation, with high yields and diastereoselectivities (more than 20:1 dl:meso). An enantioselective version (up to 92% e.e.), using catalytic amounts of a chiral titanium salen, was also developed.[6]
Two examples of pinacol coupling used in total synthesis are the Mukaiyama Taxol total synthesis and the Nicolaou Taxol total synthesis.
p-Hydroxypropiophenone is used as the substrate in the synthesis of diethylstilbestrol.
An unsymmetrical pinacol coupling reaction between para-chloro-acetophenone and acetone was employed to give phenaglycodol in a 40% yield.
References
- ↑ "Ueber einige Producte der trockenen Destillation essigsaurer Salze" (in German). Justus Liebigs Annalen der Chemie 110: 23–45. 1859. doi:10.1002/jlac.18591100103. https://zenodo.org/record/1427129.
- ↑ "Benzopinacol". Organic Syntheses. 1943. http://www.orgsyn.org/demo.aspx?prep=cv2p0071.; Collective Volume, 2, pp. 71
- ↑ "Vanadium-catalyzed pinacol coupling reaction in water". The Journal of Organic Chemistry 70 (21): 8594–8596. October 2005. doi:10.1021/jo051213f. PMID 16209617.
- ↑ "Pinacolization of aromatic aldehydes using Zn/montmorillonite K10-ZnCl2 in aqueous THF under ultrasound". Chemical Journal on Internet 5 (1): 8. 2003. http://www.chemistrymag.org/cji/2003/051008ne.htm.
- ↑ "Asymmetric synthesis by stereocontrol". Pure and Applied Chemistry 73 (2): 283–286. 2001. doi:10.1351/pac200173020283.
- ↑ "Diastereoselective and enantioselective photoredox pinacol coupling promoted by titanium complexes with a red-absorbing organic dye". Chemical Science 13 (20): 5973–5981. May 2022. doi:10.1039/D2SC00800A. PMID 35685797.
Further reading
- "Pinacol Hydrate". Organic Syntheses. 1941. http://www.orgsyn.org/demo.aspx?prep=cv1p0459.; Collective Volume, 1, pp. 459
 |